Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms
- PMID: 16179362
- PMCID: PMC1464240
- DOI: 10.1113/jphysiol.2005.097642
Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms
Abstract
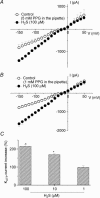
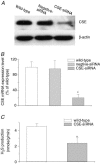
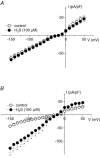
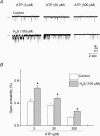
H2S is an important gasotransmitter, generated in mammalian cells from L-cysteine metabolism. As it stimulates K(ATP) channels in vascular smooth muscle cells, H2S may also function as an endogenous opener of K(ATP) channels in INS-1E cells, an insulin-secreting cell line. In the present study, K(ATP) channel currents in INS-1E cells were recorded using the whole-cell and single-channel recording configurations of the patch-clamp technique. K(ATP) channels in INS-1E cells have a single-channel conductance of 78 pS. These channels were activated by diazoxide and inhibited by gliclazide. ATP (3 mm) in the pipette solution inhibited K(ATP) channels in INS-1E cells. Significant amount of H2S was produced from INS-1E cells in which the expression of cystathinonie gamma-lyase (CSE) was confirmed. After INS-1E cells were transfected with CSE-targeted short interfering RNA (CSE-siRNA) or treated with DL-propargylglycine (PPG; 1-5 mm) to inhibit CSE, endogenous production of H2S was abolished. Increase in extracellular glucose concentration significantly decreased endogenous production of H2S in INS-1E cells, and increased insulin secretion. After transfection of INS-1E cells with adenovirus containing the CSE gene (Ad-CSE) to overexpress CSE, high glucose-stimulated insulin secretion was virtually abolished. Basal K(ATP) channel currents were significantly reduced after incubating INS-1E cells with a high glucose concentration (16 mm) or lowering endogenous H2S level by CSE-siRNA transfection. Under these conditions, exogenously applied H2S significantly increased whole-cell K(ATP) channel currents at concentrations equal to or lower than 100 microm. H2S (100 microm) markedly increased open probability by more than 2-fold of single K(ATP) channels (inside-out recording) in native INS-1E cells (n = 4, P < 0.05). Single-channel conductance and ATP sensitivity of K(ATP) channels were not changed by H2S. In conclusion, endogenous H2S production from INS-1E cells varies with in vivo conditions, which significantly affects insulin secretion from INS-1E cells. H2S stimulates K(ATP) channels in INS-1E cells, independent of activation of cytosolic second messengers, which may underlie H2S-inhibited insulin secretion from these cells. Interaction among H2S, glucose and the K(ATP) channel may constitute an important and novel mechanism for the fine control of insulin secretion from pancreatic beta-cells.
Figures





References
-
- Ashcroft FM. Mechanism of the glycaemic effects of sulfonylureas. Horm Metab Res. 1996;28:456–463. - PubMed
-
- Ashcroft SJH. The β-cell KATP channel. J Membr Bio. 2000;176:187–206. - PubMed
-
- Ashcroft FM, Gribble FM. Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci. 1998;21:288–294. - PubMed
-
- Ashcroft FM, Rorsman P, Trube G. Single calcium channel activity in mouse pancreatic beta-cells. Ann N Y Acad Sci. 1989;560:410–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

