Protein kinase C shifts the voltage dependence of KCNQ/M channels expressed in Xenopus oocytes
- PMID: 16179364
- PMCID: PMC1464213
- DOI: 10.1113/jphysiol.2005.094995
Protein kinase C shifts the voltage dependence of KCNQ/M channels expressed in Xenopus oocytes
Abstract
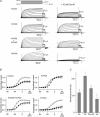
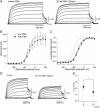
It is well established that stimulation of G(q)-coupled receptors such as the M1 muscarinic acetylcholine receptor inhibits KCNQ/M currents. While it is generally accepted that this muscarinic inhibition is mainly caused by the breakdown of PIP(2), the role of the subsequent activation of protein kinase C (PKC) is not well understood. By reconstituting M currents in Xenopus oocytes, we observed that stimulation of coexpressed M1 receptors with 10 microm oxotremorine M (oxo-M) induces a positive shift (4-30 mV, depending on which KCNQ channels are expressed) in the conductance-voltage relationship (G-V) of KCNQ channels. When we applied phorbol 12-myristate 13-acetate (PMA), a potent PKC activator, we observed a large positive shift (17.8 +/- 1.6 mV) in the G-V curve for KCNQ2, while chelerythrine, a PKC inhibitor, attenuated the shift caused by the stimulation of M1 receptors. By contrast, reducing PIP(2) had little effect on the G-V curve for KCNQ2 channels; although pretreating cells with 10 mum wortmannin for 30 min reduced KCNQ2 current amplitude by 80%, the G-V curve was shifted only slightly (5 mV). Apparently, the shift induced by muscarinic stimulation in Xenopus oocytes was mainly caused by PKC activation. When KCNQ2/3 channels were expressed in HEK 293T cells, the G-V curve seemed already to be shifted in a positive direction, even before activation of PKC, and PMA failed to shift the curve any further. That alkaline phosphatase in the patch pipette shifted the G-V curve in a negative direction suggests KCNQ2/3 channels are constitutively phosphorylated in HEK 293T cells.
Figures






Similar articles
-
A novel muscarinic receptor-independent mechanism of KCNQ2/3 potassium channel blockade by Oxotremorine-M.Eur J Pharmacol. 2016 Nov 15;791:221-228. doi: 10.1016/j.ejphar.2016.08.037. Epub 2016 Aug 31. Eur J Pharmacol. 2016. PMID: 27590358
-
Analysis of the effects of halothane on Gi-coupled muscarinic M2 receptor signaling in Xenopus oocytes using a chimeric G alpha protein.Pharmacology. 2004 Nov;72(3):205-12. doi: 10.1159/000080106. Pharmacology. 2004. PMID: 15452370
-
Effects of volatile anesthetics on the direct and indirect protein kinase C-mediated enhancement of alpha1E-type Ca(2+) current in Xenopus oocytes.J Pharmacol Exp Ther. 2000 May;293(2):360-9. J Pharmacol Exp Ther. 2000. PMID: 10773003
-
KCNQ potassium channels in sensory system and neural circuits.Acta Pharmacol Sin. 2016 Jan;37(1):25-33. doi: 10.1038/aps.2015.131. Epub 2015 Dec 21. Acta Pharmacol Sin. 2016. PMID: 26687932 Free PMC article. Review.
-
Kv7 channels: interaction with dopaminergic and serotonergic neurotransmission in the CNS.J Physiol. 2008 Apr 1;586(7):1823-32. doi: 10.1113/jphysiol.2007.149450. Epub 2008 Jan 3. J Physiol. 2008. PMID: 18174210 Free PMC article. Review.
Cited by
-
The neuronal serum- and glucocorticoid-regulated kinase 1.1 reduces neuronal excitability and protects against seizures through upregulation of the M-current.J Neurosci. 2013 Feb 6;33(6):2684-96. doi: 10.1523/JNEUROSCI.3442-12.2013. J Neurosci. 2013. PMID: 23392695 Free PMC article.
-
Does diacylglycerol regulate KCNQ channels?Pflugers Arch. 2006 Dec;453(3):293-301. doi: 10.1007/s00424-006-0092-3. Epub 2006 May 24. Pflugers Arch. 2006. PMID: 16721610
-
Protein kinase C activity is a protective modifier of Purkinje neuron degeneration in cerebellar ataxia.Hum Mol Genet. 2018 Apr 15;27(8):1396-1410. doi: 10.1093/hmg/ddy050. Hum Mol Genet. 2018. PMID: 29432535 Free PMC article.
-
Differential protein kinase C-dependent modulation of Kv7.4 and Kv7.5 subunits of vascular Kv7 channels.J Biol Chem. 2014 Jan 24;289(4):2099-111. doi: 10.1074/jbc.M113.527820. Epub 2013 Dec 2. J Biol Chem. 2014. PMID: 24297175 Free PMC article.
-
Second coiled-coil domain of KCNQ channel controls current expression and subfamily specific heteromultimerization by salt bridge networks.J Physiol. 2008 Jun 15;586(12):2827-40. doi: 10.1113/jphysiol.2007.148601. Epub 2008 Apr 25. J Physiol. 2008. PMID: 18440995 Free PMC article.
References
-
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

