Rapid directional shift of mitochondrial DNA heteroplasmy in animal tissues by a mitochondrially targeted restriction endonuclease
- PMID: 16179392
- PMCID: PMC1242285
- DOI: 10.1073/pnas.0502896102
Rapid directional shift of mitochondrial DNA heteroplasmy in animal tissues by a mitochondrially targeted restriction endonuclease
Abstract
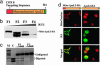
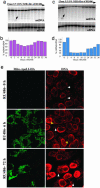
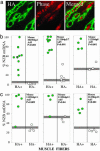
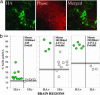
Frequently, mtDNA with pathogenic mutations coexist with wild-type genomes (mtDNA heteroplasmy). Mitochondrial dysfunction and disease ensue only when the proportion of mutated mtDNAs is high, thus a reduction in this proportion should provide an effective therapy for these disorders. We developed a system to decrease specific mtDNA haplotypes by expressing a mitochondrially targeted restriction endonuclease, ApaLI, in cells of heteroplasmic mice. These mice have two mtDNA haplotypes, of which only one contains an ApaLI site. After transfection of cultured hepatocytes with mitochondrially targeted ApaLI, we found a rapid, directional, and complete shift in mtDNA heteroplasmy (2-6 h). We tested the efficacy of this approach in vivo, by using recombinant viral vectors expressing the mitochondrially targeted ApaLI. We observed a significant shift in mtDNA heteroplasmy in muscle and brain transduced with recombinant viruses. This strategy could prevent disease onset or reverse clinical symptoms in patients harboring certain heteroplasmic pathogenic mutations in mtDNA.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

