Xanthorhodopsin: a proton pump with a light-harvesting carotenoid antenna
- PMID: 16179480
- PMCID: PMC3065861
- DOI: 10.1126/science.1118046
Xanthorhodopsin: a proton pump with a light-harvesting carotenoid antenna
Abstract
Energy transfer from light-harvesting carotenoids to chlorophyll is common in photosynthesis, but such antenna pigments have not been observed in retinal-based ion pumps and photoreceptors. Here we describe xanthorhodopsin, a proton-pumping retinal protein/carotenoid complex in the eubacterium Salinibacter ruber. The wavelength dependence of the rate of pumping and difference absorption spectra measured under a variety of conditions indicate that this protein contains two chromophores, retinal and the carotenoid salinixanthin, in a molar ratio of about 1:1. The two chromophores interact strongly, and light energy absorbed by the carotenoid is transferred to the retinal with a quantum efficiency of approximately 40%. The antenna carotenoid extends the wavelength range of the collection of light for uphill transmembrane proton transport.
Figures
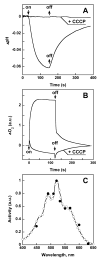
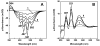
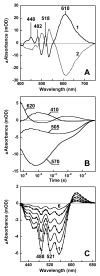
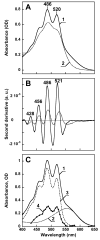
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

