Negative regulation of myofibroblast differentiation by PTEN (Phosphatase and Tensin Homolog Deleted on chromosome 10)
- PMID: 16179636
- PMCID: PMC1434700
- DOI: 10.1164/rccm.200507-1058OC
Negative regulation of myofibroblast differentiation by PTEN (Phosphatase and Tensin Homolog Deleted on chromosome 10)
Abstract
Rationale: Myofibroblasts are primary effector cells in idiopathic pulmonary fibrosis (IPF). Defining mechanisms of myofibroblast differentiation may be critical to the development of novel therapeutic agents.
Objective: To show that myofibroblast differentiation is regulated by phosphatase and tensin homolog deleted on chromosome 10 (PTEN) activity in vivo, and to identify a potential mechanism by which this occurs.
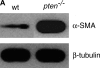
Methods: We used tissue sections of surgical lung biopsies from patients with IPF to localize expression of PTEN and alpha-smooth muscle actin (alpha-SMA). We used cell culture of pten(-/-) and wild-type fibroblasts, as well as adenoviral strategies and pharmacologic inhibitors, to determine the mechanism by which PTEN inhibits alpha-SMA, fibroblast proliferation, and collagen production.
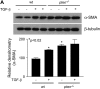
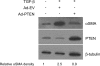
Results: In human lung specimens of IPF, myofibroblasts within fibroblastic foci demonstrated diminished PTEN expression. Furthermore, inhibition of PTEN in mice worsened bleomycin-induced fibrosis. In pten(-/-) fibroblasts, and in normal fibroblasts in which PTEN was inhibited, alpha-SMA, proliferation, and collagen production was upregulated. Addition of transforming growth factor-beta to wild-type cells, but not pten(-/-) cells, resulted in increased alpha-SMA expression in a time-dependent fashion. In pten(-/-) cells, reconstitution of PTEN decreased alpha-SMA expression, proliferation, and collagen production, whereas overexpression of PTEN in wild-type cells inhibited transforming growth factor-beta-induced myofibroblast differentiation. It was observed that both the protein and lipid phosphatase actions of PTEN were capable of modulating the myofibroblast phenotype.
Conclusions: The results indicate that in IPF, myofibroblasts have diminished PTEN expression. Inhibition of PTEN in vivo promotes fibrosis, and PTEN inhibits myofibroblast differentiation in vitro.
Figures




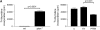
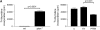
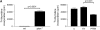







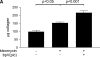
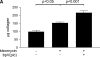
Comment in
-
PTEN as a new agent in the fight against fibrogenesis.Am J Respir Crit Care Med. 2006 Jan 1;173(1):5-6. doi: 10.1164/rccm.2510001. Am J Respir Crit Care Med. 2006. PMID: 16368792 No abstract available.
References
-
- Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971;27:549–550. - PubMed
-
- Zhang K, Gharaee-Kermani M, McGarry B, Phan SH. In situ hybridization analysis of rat lung alpha 1(I) and alpha 2(I) collagen gene expression in pulmonary fibrosis induced by endotracheal bleomycin injection. Lab Invest 1994;70:192–202. - PubMed
-
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4, 5-trisphosphate. J Biol Chem 1998;273:13375–13378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

