Screening of crystallin-crystallin interactions using microequilibrium dialysis
- PMID: 16179909
- PMCID: PMC1361693
Screening of crystallin-crystallin interactions using microequilibrium dialysis
Abstract
Purpose: It has been hypothesized that short-range, protein-protein interactions of crystallin are necessary for the maintenance of lens transparency. Because of their probable weak nature, it has been difficult to both detect and quantitate the nature of these interactions. To determine if interactions exist between alpha-crystallin and gamma-crystallin under true equilibrium conditions, we have used microequilibrium dialysis.
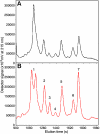
Methods: Total alpha-crystallin and gamma-crystallin were prepared from soluble proteins of fetal bovine lenses by HPLC and gel filtration chromatography. The proteins were added to one side of a microequilibrium dialysis cell, comprised of two chambers separated by a membrane with 100 kDa molecular weight cut-off. After reaching equilibrium, the amount of free gamma-crystallin and the amount of gamma-crystallin bound to alpha-crystallin was determined by HPLC and reverse phase analysis of both chambers. Selected gamma-crystallin that bound to alpha-crystallin was further purified by ion exchange chromatography, and then incubated with alpha-crystallin, to verify the specificity of their binding.
Results: Analysis of both microequilibrium dialysis chambers incubated at different times at 37 degrees C indicated that equilibrium was reached at 4 days. When total alpha-crystallin and gamma-crystallin were incubated for this time period, significant binding was observed between alpha-crystallin and the IIIA, II, and IVA species of gamma-crystallin. These interactions were confirmed by microequilibrium dialysis determinations containing alpha-crystallin and purified gamma-crystallin species.
Conclusions: These results show that microequilibrium dialysis can be used to demonstrate significant noncovalent interactions of alpha-crystallin and gamma-crystallin under true equilibrium conditions.
Figures



Similar articles
-
Age-dependent association of gamma-crystallins with aged alpha-crystallins from old bovine lens.Mol Vis. 2008 May 19;14:970-4. Mol Vis. 2008. PMID: 18509547 Free PMC article.
-
Decreased association of aged alpha crystallins with gamma crystallins.Exp Eye Res. 2006 Oct;83(4):793-7. doi: 10.1016/j.exer.2006.03.020. Epub 2006 May 18. Exp Eye Res. 2006. PMID: 16712838
-
Interaction of lens alpha and gamma crystallins during aging of the bovine lens.Exp Eye Res. 2005 Dec;81(6):680-9. doi: 10.1016/j.exer.2005.04.006. Epub 2005 Jun 20. Exp Eye Res. 2005. PMID: 15967431
-
Isolation and characterization of betaA3-crystallin associated proteinase from alpha-crystallin fraction of human lenses.Mol Vis. 2008;14:1872-85. Epub 2008 Oct 20. Mol Vis. 2008. PMID: 18949065 Free PMC article.
-
Crosslinking of human lens 9 kDa gammaD-crystallin fragment in vitro and in vivo.Mol Vis. 2003 Dec 8;9:644-56. Mol Vis. 2003. PMID: 14685148
Cited by
-
Interaction of βA3-Crystallin with Deamidated Mutants of αA- and αB-Crystallins.PLoS One. 2015 Dec 11;10(12):e0144621. doi: 10.1371/journal.pone.0144621. eCollection 2015. PLoS One. 2015. PMID: 26657544 Free PMC article.
-
Age-dependent association of gamma-crystallins with aged alpha-crystallins from old bovine lens.Mol Vis. 2008 May 19;14:970-4. Mol Vis. 2008. PMID: 18509547 Free PMC article.
-
Protein-protein interactions involving congenital cataract T5P gammaC-crystallin mutant: a confocal fluorescence microscopy study.Exp Eye Res. 2008 Dec;87(6):515-20. doi: 10.1016/j.exer.2008.08.021. Epub 2008 Sep 26. Exp Eye Res. 2008. PMID: 18926820 Free PMC article.
-
Protein-protein interactions and lens transparency.Exp Eye Res. 2008 Dec;87(6):496-501. doi: 10.1016/j.exer.2008.08.018. Epub 2008 Sep 18. Exp Eye Res. 2008. PMID: 18835387 Free PMC article. Review.
-
Identification of interaction sites between human betaA3- and alphaA/alphaB-crystallins by mammalian two-hybrid and fluorescence resonance energy transfer acceptor photobleaching methods.J Biol Chem. 2009 Jul 3;284(27):18481-92. doi: 10.1074/jbc.M109.013789. Epub 2009 Apr 28. J Biol Chem. 2009. PMID: 19401464 Free PMC article.
References
-
- Bloemendal H. The vertebrate eye lens. Science. 1977;197:127–38. - PubMed
-
- Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–7. - PubMed
-
- Mach H, Trautman PA, Thomson JA, Lewis RV, Middaugh CR. Inhibition of alpha-crystallin aggregation by gamma-crystallin. J Biol Chem. 1990;265:4844–8. - PubMed
-
- Fu L, Liang JJ. Detection of protein-protein interactions among lens crystallins in a mammalian two-hybrid system assay. J Biol Chem. 2002;277:4255–60. - PubMed
-
- Fu L, Liang JJ. Alteration of protein-protein interactions of congenital cataract crystallin mutants. Invest Ophthalmol Vis Sci. 2003;44:1155–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

