Employing new cellular therapeutic targets for Alzheimer's disease: a change for the better?
- PMID: 16181100
- PMCID: PMC2254177
- DOI: 10.2174/1567202052773508
Employing new cellular therapeutic targets for Alzheimer's disease: a change for the better?
Abstract
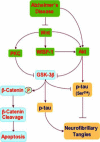
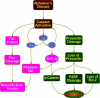
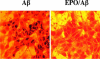
Alzheimer's disease is a progressive disorder that results in the loss of cognitive function and memory. Although traditionally defined by the presence of extracellular plaques of amyloid-beta peptide aggregates and intracellular neurofibrillary tangles in the brain, more recent work has begun to focus on elucidating the complexities of Alzheimer's disease that involve the generation of reactive oxygen species and oxidative stress. Apoptotic processes that are incurred as a function of oxidative stress affect neuronal, vascular, and monocyte derived cell populations. In particular, it is the early apoptotic induction of cellular membrane asymmetry loss that drives inflammatory microglial activation and subsequent neuronal and vascular injury. In this article, we discuss the role of novel cellular pathways that are invoked during oxidative stress and may potentially mediate apoptotic injury in Alzheimer's disease. Ultimately, targeting new avenues for the development of therapeutic strategies linked to mechanisms that involve inflammatory microglial activation, cellular metabolism, cell-cycle regulation, G-protein regulated receptors, and cytokine modulation may provide fruitful gains for both the prevention and treatment of Alzheimer's disease.
Figures
Similar articles
-
Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer's disease.Brain Res Brain Res Rev. 2005 Jul;49(1):1-21. doi: 10.1016/j.brainresrev.2004.11.005. Epub 2005 Jan 8. Brain Res Brain Res Rev. 2005. PMID: 15960984 Free PMC article. Review.
-
[Alzheimer's disease: new prospects in therapy and applied experimental models].Postepy Hig Med Dosw (Online). 2008 Aug 5;62:372-92. Postepy Hig Med Dosw (Online). 2008. PMID: 18688208 Review. Polish.
-
The modulation of metal bio-availability as a therapeutic strategy for the treatment of Alzheimer's disease.FEBS J. 2007 Aug;274(15):3775-83. doi: 10.1111/j.1742-4658.2007.05918.x. Epub 2007 Jul 6. FEBS J. 2007. PMID: 17617225 Review.
-
[Mechanisms of neurodegeneration in Alzheimer's disease].Med Pregl. 2012 Jul-Aug;65(7-8):301-7. doi: 10.2298/mpns1208301j. Med Pregl. 2012. PMID: 22924250 Review. Serbian.
-
Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease.Prog Neurobiol. 2005 Feb;75(3):207-46. doi: 10.1016/j.pneurobio.2005.02.004. Epub 2005 Apr 26. Prog Neurobiol. 2005. PMID: 15882775 Review.
Cited by
-
Erythropoietin: new directions for the nervous system.Int J Mol Sci. 2012;13(9):11102-11129. doi: 10.3390/ijms130911102. Epub 2012 Sep 6. Int J Mol Sci. 2012. PMID: 23109841 Free PMC article. Review.
-
The effect of regular exercise on antioxidant enzyme activities and lipid peroxidation levels in both hippocampi after occluding one carotid in rat.J Physiol Sci. 2014 Sep;64(5):325-32. doi: 10.1007/s12576-014-0322-y. Epub 2014 Jun 13. J Physiol Sci. 2014. PMID: 24923383 Free PMC article.
-
The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through AKT, BAD, PARP, and mitochondrial associated "anti-apoptotic" pathways.Curr Neurovasc Res. 2005 Oct;2(4):271-85. doi: 10.2174/156720205774322584. Curr Neurovasc Res. 2005. PMID: 16181120 Free PMC article.
-
Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity.Curr Neurovasc Res. 2005 Dec;2(5):387-99. doi: 10.2174/156720205774962683. Curr Neurovasc Res. 2005. PMID: 16375720 Free PMC article.
-
New strategies for Alzheimer's disease and cognitive impairment.Oxid Med Cell Longev. 2009 Nov-Dec;2(5):279-89. doi: 10.4161/oxim.2.5.9990. Oxid Med Cell Longev. 2009. PMID: 20716915 Free PMC article. Review.
References
-
- Adamec E, Mohan P, Vonsattel JP, Nixon RA. Calpain activation in neurodegenerative diseases: confocal immunofluorescence study with antibodies specifically recognizing the active form of calpain 2. Acta. Neuropathol. (Berl) 2002;104(1):92–104. - PubMed
-
- Adams S, Green P, Claxton R, Simcox S, Williams MV, Walsh K, Leeuwenburgh C. Reactive carbonyl formation by oxidative and non-oxidative pathways. Front. Biosci. 2001;6:A17–24. - PubMed
-
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. Jama. 2003;289(21):2819–26. - PubMed
-
- Altomare DA, Guo K, Cheng JQ, Sonoda G, Walsh K, Testa JR. Cloning, chromosomal localization and expression analysis of the mouse Akt2 oncogene. Oncogene. 1995;11(6):1055–60. - PubMed
-
- Alvarez A, Munoz JP, Maccioni RB. A Cdk5-p35 stable complex is involved in the beta-amyloid-induced deregulation of Cdk5 activity in hippocampal neurons. Exp. Cell. Res. 2001;264(2):266–74. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
