Netrin induces down-regulation of its receptor, Deleted in Colorectal Cancer, through the ubiquitin-proteasome pathway in the embryonic cortical neuron
- PMID: 16181408
- PMCID: PMC2683579
- DOI: 10.1111/j.1471-4159.2005.03314.x
Netrin induces down-regulation of its receptor, Deleted in Colorectal Cancer, through the ubiquitin-proteasome pathway in the embryonic cortical neuron
Abstract
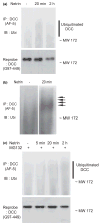
The proper regulation of temporal and spatial expression of the axon guidance cues and their receptors is critical for the normal wiring of nervous system during development. Netrins, a family of secreted guidance cues, are involved in the midline crossing of spinal commissural axons and in the guidance of cortical efferents. Axons normally lose the responsiveness to their attractants when they arrive at their targets, where the attractant is produced. However the molecular mechanism is still unknown. We investigated the molecular mechanism of down-regulation of netrin-1 signaling in the embryonic cortical neurons. Netrin-1 induced the ubiquitination and proteolytic cleavage of Deleted in Colorectal Cancer (DCC), a transmembrane receptor for netrin, in dissociated cortical neurons. A dramatic decrease of DCC level particularly on the cell surface was also observed after netrin-1 stimulation. Specific ubiquitin-proteasome inhibitors prevented the netrin-induced DCC cleavage and decrease of cell surface DCC. We suggest that the ligand-mediated down-regulation of DCC might participate in the loss of netrin-responsiveness in the developing nervous system.
Figures



References
-
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. - PubMed
-
- Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron. 1997;19:575–589. - PubMed
-
- Della NG, Senior PV, Bowtell DD. Isolation and characterisation of murine homologues of the Drosophila seven in absentia gene (sina) Development. 1993;117:1333–1343. - PubMed
-
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. - PubMed
-
- Fazeli A, Dickinson SL, Hermiston ML, et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

