The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway
- PMID: 16183851
- PMCID: PMC1256006
- DOI: 10.1104/pp.105.061382
The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway
Abstract
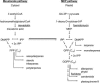
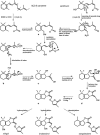
The seeds of parasitic plants of the genera Striga and Orobanche will only germinate after induction by a chemical signal exuded from the roots of their host. Up to now, several of these germination stimulants have been isolated and identified in the root exudates of a series of host plants of both Orobanche and Striga spp. In most cases, the compounds were shown to be isoprenoid and belong to one chemical class, collectively called the strigolactones, and suggested by many authors to be sesquiterpene lactones. However, this classification was never proven; hence, the biosynthetic pathways of the germination stimulants are unknown. We have used carotenoid mutants of maize (Zea mays) and inhibitors of isoprenoid pathways on maize, cowpea (Vigna unguiculata), and sorghum (Sorghum bicolor) and assessed the effects on the root exudate-induced germination of Striga hermonthica and Orobanche crenata. Here, we show that for these three host and two parasitic plant species, the strigolactone germination stimulants are derived from the carotenoid pathway. Furthermore, we hypothesize how the germination stimulants are formed. We also discuss this finding as an explanation for some phenomena that have been observed for the host-parasitic plant interaction, such as the effect of mycorrhiza on S. hermonthica infestation.
Figures





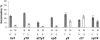
Similar articles
-
Carotenoid inhibitors reduce strigolactone production and Striga hermonthica infection in rice.Arch Biochem Biophys. 2010 Dec 1;504(1):123-31. doi: 10.1016/j.abb.2010.08.005. Epub 2010 Aug 21. Arch Biochem Biophys. 2010. PMID: 20732294
-
Strigol: biogenesis and physiological activity.Phytochemistry. 2006 Apr;67(7):636-40. doi: 10.1016/j.phytochem.2005.12.026. Epub 2006 Feb 17. Phytochemistry. 2006. PMID: 16483620 Review.
-
Strigolactone analogs derived from ketones using a working model for germination stimulants as a blueprint.Plant Cell Physiol. 2011 Apr;52(4):699-715. doi: 10.1093/pcp/pcr031. Epub 2011 Mar 18. Plant Cell Physiol. 2011. PMID: 21421570
-
Strigolactones and root infestation by plant-parasitic Striga, Orobanche and Phelipanche spp.Plant Sci. 2011 Mar;180(3):414-20. doi: 10.1016/j.plantsci.2010.11.007. Epub 2010 Nov 23. Plant Sci. 2011. PMID: 21421387 Review.
-
Synthesis and bioactivity of labelled germination stimulants for the isolation and identification of the strigolactone receptor.Org Biomol Chem. 2003 Mar 21;1(6):950-9. doi: 10.1039/b210678g. Org Biomol Chem. 2003. PMID: 12929633
Cited by
-
The role of strigolactones in nutrient-stress responses in plants.Int J Mol Sci. 2013 Apr 29;14(5):9286-304. doi: 10.3390/ijms14059286. Int J Mol Sci. 2013. PMID: 23629665 Free PMC article. Review.
-
Global Transcriptomic Analysis Reveals the Mechanism of Phelipanche aegyptiaca Seed Germination.Int J Mol Sci. 2016 Jul 15;17(7):1139. doi: 10.3390/ijms17071139. Int J Mol Sci. 2016. PMID: 27428962 Free PMC article.
-
Ha-DEF1, a sunflower defensin, induces cell death in Orobanche parasitic plants.Planta. 2007 Aug;226(3):591-600. doi: 10.1007/s00425-007-0507-1. Epub 2007 Mar 21. Planta. 2007. PMID: 17375322
-
Role of carotenoid cleavage dioxygenase 1 (CCD1) in apocarotenoid biogenesis revisited.Plant Signal Behav. 2009 Mar;4(3):172-5. doi: 10.4161/psb.4.3.7840. Plant Signal Behav. 2009. PMID: 19721743 Free PMC article. Review.
-
Strigolactones GR-24 and Nijmegen Applications Result in Reduced Susceptibility of Tobacco and Grapevine Plantlets to Botrytis cinerea Infection.Plants (Basel). 2023 Sep 7;12(18):3202. doi: 10.3390/plants12183202. Plants (Basel). 2023. PMID: 37765366 Free PMC article.
References
-
- Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827 - PubMed
-
- Ben-Amotz A, Fishler R (1998) Analysis of carotenoids with emphasis on 9-cis beta-carotene in vegetables and fruits commonly consumed in Israel. Food Chem 62: 515–520
-
- Bino RJ, de Vos RCH, Lieberman M, Hall RD, Bovy A, Jonker HH, Tikunov Y, Lommen A, Moco S, Levin I (2005) The light-hyperresponsive high pigment-2dg mutation of tomato: alterations in the fruit metabolome. New Phytol 166: 427–438 - PubMed
-
- Botella-Pavia P, Besumbes O, Phillips MA, Carretero-Paulet L, Boronat A, Rodriguez-Concepcion M (2004) Regulation of carotenoid biosynthesis in plants: evidence for a key role of hydroxymethylbutenyl diphosphate reductase in controlling the supply of plastidial isoprenoid precursors. Plant J 40: 188–199 - PubMed
-
- Bouwmeester HJ, Matusova R, Zhongkui S, Beale MH (2003) Secondary metabolite signalling in host-parasitic plant interactions. Curr Opin Plant Biol 6: 358–364 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

