Cadherin mechanics and complexation: the importance of calcium binding
- PMID: 16183887
- PMCID: PMC1366956
- DOI: 10.1529/biophysj.105.067322
Cadherin mechanics and complexation: the importance of calcium binding
Abstract
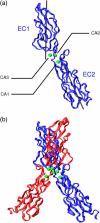
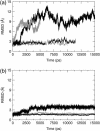
E-cadherins belong to a family of membrane-bound, cellular adhesion proteins. Their adhesive properties mainly involve the two N-terminal extracellular domains (EC1 and EC2). The junctions between these domains are characterized by calcium ion binding sites, and calcium ions are essential for the correct functioning of E-cadherins. Calcium is believed to rigidify the extracellular portion of the protein, which, when complexed, adopts a rod-like conformation. Here, we use molecular dynamics simulations to investigate the dynamics of the EC1-2 portion of E-cadherin in the presence and in the absence of calcium ions. These simulations confirm that apo-cadherin shows much higher conformational flexibility on a nanosecond timescale than the calcium-bound form. It is also shown that although the apo-cadherin fragment can spontaneously complex potassium, these monovalent ions are incapable of rigidifying the interdomain junctions. In contrast, removal of the most solvent-exposed calcium ion at the EC1-2 junction does not significantly perturb the dynamical behavior of the fragment. We have also extended this study to the cis-dimer formed from two EC1-2 fragments, potentially involved in cellular adhesion. Here again, it is shown that the presence of calcium is an important factor in both rigidifying and stabilizing the complex.
Figures







References
-
- Yap, A. S., W. M. Brieher, and B. M. Gumbiner. 1997. Molecular and functional analysis of cadherin-based adherens junctions. Annu. Rev. Cell Dev. Biol. 13:119–146. - PubMed
-
- Takeichi, M. 1990. Cadherins: a molecular family important in selective cell-cell adhesion. Annu. Rev. Biochem. 59:237–252. - PubMed
-
- Patel, S. D., C. P. Chen, F. Bahna, B. Honig, and L. Shapiro. 2003. Cadherin-mediated cell-cell adhesion: sticking together as a family. Curr. Opin. Struct. Biol. 13:690–698. - PubMed
-
- Handschuh, G., B. Luber, P. Hutzler, H. Hofler, and K. F. Becker. 2001. Single amino acid substitutions in conserved extracellular domains of E-cadherin differ in their functional consequences. J. Mol. Biol. 314:445–454. - PubMed
-
- Alattia, J. R., J. B. Ames, T. Porumb, K. I. Tong, Y. M. Heng, P. Ottensmeyer, C. M. Kay, and M. Ikura. 1997. Lateral self-assembly of E-cadherin directed by cooperative calcium binding. FEBS Lett. 417:405–408. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

