Properties and structures of the influenza and HIV fusion peptides on lipid membranes: implications for a role in fusion
- PMID: 16183890
- PMCID: PMC1366814
- DOI: 10.1529/biophysj.105.063032
Properties and structures of the influenza and HIV fusion peptides on lipid membranes: implications for a role in fusion
Abstract
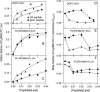
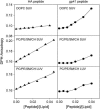
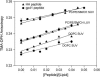
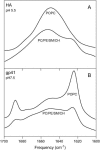
The fusion peptides of HIV and influenza virus are crucial for viral entry into a host cell. We report the membrane-perturbing and structural properties of fusion peptides from the HA fusion protein of influenza virus and the gp41 fusion protein of HIV. Our goals were to determine: 1), how fusion peptides alter structure within the bilayers of fusogenic and nonfusogenic lipid vesicles and 2), how fusion peptide structure is related to the ability to promote fusion. Fluorescent probes revealed that neither peptide had a significant effect on bilayer packing at the water-membrane interface, but both increased acyl chain order in both fusogenic and nonfusogenic vesicles. Both also reduced free volume within the bilayer as indicated by partitioning of a lipophilic fluorophore into membranes. These membrane ordering effects were smaller for the gp41 peptide than for the HA peptide at low peptide/lipid ratio, suggesting that the two peptides assume different structures on membranes. The influenza peptide was predominantly helical, and the gp41 peptide was predominantly antiparallel beta-sheet when membrane bound, however, the depths of penetration of Trps of both peptides into neutral membranes were similar and independent of membrane composition. We previously demonstrated: 1), the abilities of both peptides to promote fusion but not initial intermediate formation during PEG-mediated fusion and 2), the ability of hexadecane to compete with this effect of the fusion peptides. Taken together, our current and past results suggest a hypothesis for a common mechanism by which these two viral fusion peptides promote fusion.
Figures

Similar articles
-
2H nuclear magnetic resonance spectroscopy supports larger amplitude fast motion and interference with lipid chain ordering for membrane that contains β sheet human immunodeficiency virus gp41 fusion peptide or helical hairpin influenza virus hemagglutinin fusion peptide at fusogenic pH.Biochim Biophys Acta Biomembr. 2020 Oct 1;1862(10):183404. doi: 10.1016/j.bbamem.2020.183404. Epub 2020 Jun 23. Biochim Biophys Acta Biomembr. 2020. PMID: 32585207 Free PMC article.
-
Effect of the N-terminal glycine on the secondary structure, orientation, and interaction of the influenza hemagglutinin fusion peptide with lipid bilayers.Biophys J. 1996 May;70(5):2275-86. doi: 10.1016/S0006-3495(96)79793-X. Biophys J. 1996. PMID: 9172751 Free PMC article.
-
Hemagglutinin fusion peptide mutants in model membranes: structural properties, membrane physical properties, and PEG-mediated fusion.Biophys J. 2011 Sep 7;101(5):1095-104. doi: 10.1016/j.bpj.2011.07.031. Biophys J. 2011. PMID: 21889446 Free PMC article.
-
What studies of fusion peptides tell us about viral envelope glycoprotein-mediated membrane fusion (review).Mol Membr Biol. 1997 Jul-Sep;14(3):97-112. doi: 10.3109/09687689709048170. Mol Membr Biol. 1997. PMID: 9394290 Review.
-
Structural and functional specificity of Influenza virus haemagglutinin and paramyxovirus fusion protein anchoring peptides.Virus Res. 2017 Jan 2;227:183-199. doi: 10.1016/j.virusres.2016.09.014. Epub 2016 Oct 20. Virus Res. 2017. PMID: 27773768 Review.
Cited by
-
PEG as a tool to gain insight into membrane fusion.Eur Biophys J. 2007 Apr;36(4-5):315-26. doi: 10.1007/s00249-006-0097-z. Epub 2006 Oct 13. Eur Biophys J. 2007. PMID: 17039359 Review.
-
Computational methods to study enveloped viral entry.Biochem Soc Trans. 2021 Dec 17;49(6):2527-2537. doi: 10.1042/BST20210190. Biochem Soc Trans. 2021. PMID: 34783344 Free PMC article. Review.
-
Autophagy: The multi-purpose bridge in viral infections and host cells.Rev Med Virol. 2018 Jul;28(4):e1973. doi: 10.1002/rmv.1973. Epub 2018 Apr 30. Rev Med Virol. 2018. PMID: 29709097 Free PMC article. Review.
-
Lipid Selectivity of Membrane Action of the Fragments of Fusion Peptides of Marburg and Ebola Viruses.Int J Mol Sci. 2024 Sep 13;25(18):9901. doi: 10.3390/ijms25189901. Int J Mol Sci. 2024. PMID: 39337389 Free PMC article.
-
HIV gp41 fusion peptide increases membrane ordering in a cholesterol-dependent fashion.Biophys J. 2014 Jan 7;106(1):172-81. doi: 10.1016/j.bpj.2013.11.027. Biophys J. 2014. PMID: 24411249 Free PMC article.
References
-
- White, J. M. 1990. Viral and cellular membrane fusion proteins. Annu. Rev. Physiol. 52:675–697. - PubMed
-
- Dimitrov, D. S., H. Golding, and R. Blumenthal. 1991. Initial stages of HIV-1 envelope glycoprotein-mediated cell fusion monitored by a new assay based on redistribution of fluorescent dyes. AIDS Res. Hum. Retroviruses. 7:799–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

