Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation
- PMID: 16184193
- PMCID: PMC1224299
- DOI: 10.1172/JCI25256
Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation
Abstract
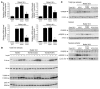
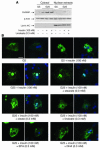
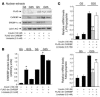
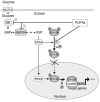
Dietary polyunsaturated fatty acids (PUFAs) are potent inhibitors of hepatic glycolysis and lipogenesis. Recently, carbohydrate-responsive element-binding protein (ChREBP) was implicated in the regulation by glucose of glycolytic and lipogenic genes, including those encoding L-pyruvate kinase (L-PK) and fatty acid synthase (FAS). The aim of our study was to assess the role of ChREBP in the control of L-PK and FAS gene expression by PUFAs. We demonstrated in mice, both in vivo and in vitro, that PUFAs [linoleate (C18:2), eicosapentanoic acid (C20:5), and docosahexaenoic acid (C22:6)] suppressed ChREBP activity by increasing ChREBP mRNA decay and by altering ChREBP translocation from the cytosol to the nucleus, independently of an activation of the AMP-activated protein kinase, previously shown to regulate ChREBP activity. In contrast, saturated [stearate (C18)] and monounsaturated fatty acids [oleate (C18:1)] had no effect. Since glucose metabolism via the pentose phosphate pathway is determinant for ChREBP nuclear translocation, the decrease in xylulose 5-phosphate concentrations caused by a PUFA diet favors a PUFA-mediated inhibition of ChREBP translocation. In addition, overexpression of a constitutive nuclear ChREBP isoform in cultured hepatocytes significantly reduced the PUFA inhibition of both L-PK and FAS gene expression. Our results demonstrate that the suppressive effect of PUFAs on these genes is primarily caused by an alteration of ChREBP nuclear translocation. In conclusion, we describe a novel mechanism to explain the inhibitory effect of PUFAs on the genes encoding L-PK and FAS and demonstrate that ChREBP is a pivotal transcription factor responsible for coordinating the PUFA suppression of glycolytic and lipogenic genes.
Figures





References
-
- Jump DB, Clarke SD. Regulation of gene expression by dietary fat. Annu. Rev. Nutr. 1999;19:63–90. - PubMed
-
- Liang G, et al. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J. Biol. Chem. 2002;277:9520–9528. - PubMed
-
- Xu J, Teran-Garcia M, Park JH, Nakamura MT, Clarke SD. Polyunsaturated fatty acids suppress hepatic sterol regulatory element-binding protein-1 expression by accelerating transcript decay. J. Biol. Chem. 2001;276:9800–9807. - PubMed
-
- Worgall TS, Sturley SL, Seo T, Osborne TF, Deckelbaum RJ. Polyunsaturated fatty acids decrease expression of promoters with sterol regulatory elements by decreasing levels of mature sterol regulatory element-binding protein. J. Biol. Chem. 1998;273:25537–25540. - PubMed
-
- Hannah VC, Ou J, Luong A, Goldstein JL, Brown MS. Unsaturated fatty acids down-regulate srebp isoforms 1a and 1c by two mechanisms in HEK-293 cells. J. Biol. Chem. 2001;276:4365–4372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

