Neurotransmitter release regulated by a MALS-liprin-alpha presynaptic complex
- PMID: 16186258
- PMCID: PMC2171538
- DOI: 10.1083/jcb.200503011
Neurotransmitter release regulated by a MALS-liprin-alpha presynaptic complex
Abstract
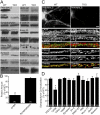
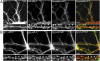
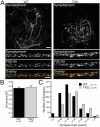
Synapses are highly specialized intercellular junctions organized by adhesive and scaffolding molecules that align presynaptic vesicular release with postsynaptic neurotransmitter receptors. The MALS/Veli-CASK-Mint-1 complex of PDZ proteins occurs on both sides of the synapse and has the potential to link transsynaptic adhesion molecules to the cytoskeleton. In this study, we purified the MALS protein complex from brain and found liprin-alpha as a major component. Liprin proteins organize the presynaptic active zone and regulate neurotransmitter release. Fittingly, mutant mice lacking all three MALS isoforms died perinatally with difficulty breathing and impaired excitatory synaptic transmission. Excitatory postsynaptic currents were dramatically reduced in autaptic cultures from MALS triple knockout mice due to a presynaptic deficit in vesicle cycling. These findings are consistent with a model whereby the MALS-CASK-liprin-alpha complex recruits components of the synaptic release machinery to adhesive proteins of the active zone.
Figures
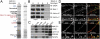


References
-
- Augustin, I., C. Rosenmund, T.C. Südhof, and N. Brose. 1999. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 400:457–461. - PubMed
-
- Borg, J.-P., Y. Yang, M. De Taddéo-Borg, B. Margolis, and R.S. Turner. 1998. The X11alpha protein slows cellular amyloid precursor protein processing and reduces Abeta40 and Abeta42 secretion. J. Biol. Chem. 273:14761–14766. - PubMed
-
- Butz, S., M. Okamoto, and T.C. Südhof. 1998. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 94:773–782. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

