Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery
- PMID: 16186323
- PMCID: PMC1987367
- DOI: 10.1167/iovs.05-0194
Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery
Abstract
Purpose: In juvenile tree shrews, a minus-power lens placed in front of the eye produces increased axial elongation and a myopic shift in refractive state that compensates for the power of the lens. Scleral tissue remodeling and modulation of the mechanical properties of the sclera occur during lens compensation. In this study, the time course of changes in scleral mRNA levels of three MMPs and three TIMPs during compensation for a minus lens and during recovery was investigated, to determine which, if any, are temporally associated with changes in the mechanical properties of the sclera and the axial elongation rate.
Methods: Competitive RT-PCR was used to measure the levels of mRNA for MT1-MMP, MMP-2, MMP-3, TIMP-1, TIMP-2, and TIMP-3 in the scleras of tree shrews that had received either 1, 2, 4, or 11 days of monocular -5-D lens treatment, or 11 days of -5-D lens treatment followed by 2 or 4 days of recovery.
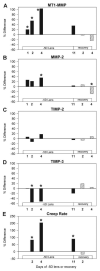
Results: Relative to their control eyes, treated eye MT1-MMP and MMP-2 mRNA levels were significantly higher, and TIMP-3 levels were lower by 1 to 4 days of minus lens treatment. These differential effects were absent by 11 days of treatment when the treated eyes had compensated for the lens. The levels of all three TIMPs spiked upward in both eyes after 2 days of recovery. The differential changes in MT1-MMP, MMP-2, and TIMP-3 mRNA levels were all restricted to the treated eye and were temporally associated with the differential changes in axial elongation, refractive state, and the previously measured changes in creep rate.
Conclusions: The observed changes in MT1-MMP, MMP-2, TIMP-2, and TIMP-3 mRNA are consistent with visually modulated MT1-MMP activation of MMP-2 and with MT1-MMP degradation of scleral extracellular matrix components. These data constitute further evidence that visual signals modulate gene expression of selected MMPs and TIMPs to control scleral remodeling, the mechanical properties of the sclera, axial elongation, and refractive state.
Figures



Similar articles
-
The time course of changes in mRNA levels in tree shrew sclera during induced myopia and recovery.Invest Ophthalmol Vis Sci. 2002 Jul;43(7):2067-75. Invest Ophthalmol Vis Sci. 2002. PMID: 12091398 Free PMC article.
-
Patterns of mRNA and protein expression during minus-lens compensation and recovery in tree shrew sclera.Mol Vis. 2011 Apr 12;17:903-19. Mol Vis. 2011. PMID: 21541268 Free PMC article.
-
Selective modulation of scleral proteoglycan mRNA levels during minus lens compensation and recovery.Mol Vis. 2007 Oct 4;13:1878-86. Mol Vis. 2007. PMID: 17960126
-
Steady state mRNA levels in tree shrew sclera with form-deprivation myopia and during recovery.Invest Ophthalmol Vis Sci. 2001 May;42(6):1153-9. Invest Ophthalmol Vis Sci. 2001. PMID: 11328721
-
Gene expression signatures in tree shrew sclera during recovery from minus-lens wear and during plus-lens wear.Mol Vis. 2019 Jun 19;25:311-328. eCollection 2019. Mol Vis. 2019. PMID: 31341380 Free PMC article.
Cited by
-
Defect of TIMP4 Is Associated with High Myopia and Participates in Rat Ocular Development in a Dose-Dependent Manner.Int J Mol Sci. 2023 Nov 29;24(23):16928. doi: 10.3390/ijms242316928. Int J Mol Sci. 2023. PMID: 38069250 Free PMC article.
-
Binocular lens treatment in tree shrews: Effect of age and comparison of plus lens wear with recovery from minus lens-induced myopia.Exp Eye Res. 2010 Nov;91(5):660-9. doi: 10.1016/j.exer.2010.08.010. Epub 2010 Aug 14. Exp Eye Res. 2010. PMID: 20713041 Free PMC article.
-
Ocular expression of avian thymic hormone: changes during the recovery from induced myopia.Mol Vis. 2009;15:778-92. Epub 2009 Apr 17. Mol Vis. 2009. PMID: 19390653 Free PMC article.
-
Altered gene expression in tree shrew retina and retinal pigment epithelium produced by short periods of minus-lens wear.Exp Eye Res. 2018 Mar;168:77-88. doi: 10.1016/j.exer.2018.01.005. Epub 2018 Jan 9. Exp Eye Res. 2018. PMID: 29329973 Free PMC article.
-
Evaluation of MMP2 as a candidate gene for high myopia.Mol Vis. 2013;19:121-7. Epub 2013 Jan 28. Mol Vis. 2013. PMID: 23378725 Free PMC article.
References
-
- Norton TT. Animal models of myopia: learning how vision controls the size of the eye. ILAR J. 1999;40:59–77. - PubMed
-
- Wildsoet CF. Active emmetropization: evidence for its existence and ramifications for clinical practice. Ophthalmic Physiol Opt. 1997;17:279–290. - PubMed
-
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. - PubMed
-
- Smith EL. Environmentally induced refractive errors in animals. In: Rosenfield M, Gilmartin B, editors. Myopia and Nearwork. Butterworth-Heinemann; Oxford, UK: 1998. pp. 57–90.
-
- Lauber JK, McGinnis J, Boyd J. Influence of mitotics, diamox and vision occluders on light-induced buphthalmos in domestic fowl. Proc Soc Exp Biol Med. 1965;120:572–575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

