A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels
- PMID: 16186483
- PMCID: PMC1234902
- DOI: 10.1073/pnas.0507170102
A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels
Abstract
Pseudomonas aeruginosa causes chronic biofilm infections, and its ability to attach to surfaces and other cells is important for biofilm formation and maintenance. Mutations in a gene called wspF, part of a putative chemosensory signal-transduction operon, have been shown to result in cell aggregation and altered colony morphology. The WspF phenotypes depend on the presence of WspR, which is a member of a family of signal transduction proteins known as response regulators. It is likely that the effect of the wspF mutation is to cause constitutive activation of WspR by phosphorylation. WspR contains a GGDEF domain known to catalyze formation of a cytoplasmic signaling molecule cyclic diguanylate (c-diGMP). We determined that purified WspR catalyzed the formation of c-diGMP in vitro and phosphorylation stimulated this activity. We observed increased cellular levels of c-diGMP and increased biofilm formation in a wspF mutant. Expression of a protein predicted to catalyze degradation of c-diGMP reversed the phenotypes of a wspF mutant and inhibited biofilm initiation by wild-type cells, indicating that the presence of c-diGMP is necessary for biofilm formation. A transcriptome analysis showed that expression levels of at least 560 genes were affected by a wspF deletion. The psl and pel operons, which are involved in exopolysaccharide production and biofilm formation, were expressed at high levels in a wspF mutant. Together, the data suggest that the wsp signal transduction pathway regulates biofilm formation through modulation of cyclic diguanylate levels.
Figures
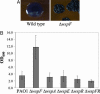
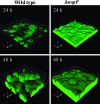

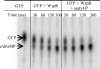

References
-
- Stewart, P. S. & Costerton, J. W. (2001) Lancet 358, 135-138. - PubMed
-
- Mah, T. F., Pitts, B., Pellock, B., Walker, G. C., Stewart, P. S. & O'Toole, G. A. (2003) Nature 426, 306-310. - PubMed
-
- Mah, T. F. & O'Toole, G. A. (2001) Trends Microbiol. 9, 34-39. - PubMed
-
- Lyczak, J. B., Cannon, C. L. & Pier, G. B. (2000) Microbes Infect. 2, 1051-1060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

