Long-term potentiation of exogenous glutamate responses at single dendritic spines
- PMID: 16186507
- PMCID: PMC1242281
- DOI: 10.1073/pnas.0501956102
Long-term potentiation of exogenous glutamate responses at single dendritic spines
Abstract
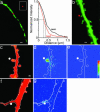
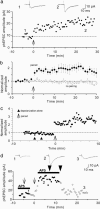
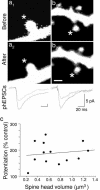
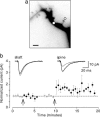
Long-term increases in the strength of excitatory transmission at Schaffer collateral-CA1 cell synapses of the hippocampus require the insertion of new alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors (AMPARs) into the synapse, but the kinetics of this process are not well established. Using microphotolysis of caged glutamate to activate receptors at single dendritic spines in hippocampal CA1 cells, we report the long-lasting potentiation of AMPAR-mediated currents with only a single pairing of photoreleased glutamate and brief postsynaptic depolarization. This potentiation was N-methyl-d-aspartate receptor (NMDAR)-dependent and was reversed with low-frequency photostimulation in an NMDAR-dependent manner, suggesting that it is mediated by the same mechanism(s) as conventional synaptic long-term potentiation. Potentiation of photolytic responses developed rapidly in a stepwise manner after a brief and variable delay (<60 s) at spines, but could not be induced at extrasynaptic sites on the dendritic shaft. Potentiation was accompanied by a concomitant decrease in postsynaptic, polyamine-dependent paired-pulse facilitation of the photolytic currents, indicating that a change in the subunit composition of the AMPARs underlying the response contributed to the potentiation. These changes are consistent with an increase in the proportion of GluR2-containing AMPARs in the spine head. These results demonstrate that activation of postsynaptic glutamate receptors by glutamate is not only necessary, but sufficient, for the induction of NMDAR-dependent long-term potentiation and reveal additional aspects of its expression.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

