Endogenous protease activation of ENaC: effect of serine protease inhibition on ENaC single channel properties
- PMID: 16186561
- PMCID: PMC2266620
- DOI: 10.1085/jgp.200509285
Endogenous protease activation of ENaC: effect of serine protease inhibition on ENaC single channel properties
Abstract
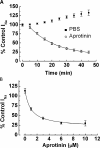
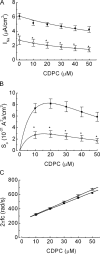
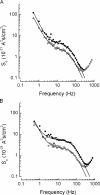
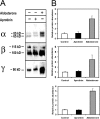
Endogenous serine proteases have been reported to control the reabsorption of Na(+) by kidney- and lung-derived epithelial cells via stimulation of electrogenic Na(+) transport mediated by the epithelial Na(+) channel (ENaC). In this study we investigated the effects of aprotinin on ENaC single channel properties using transepithelial fluctuation analysis in the amphibian kidney epithelium, A6. Aprotinin caused a time- and concentration-dependent inhibition (84 +/- 10.5%) in the amiloride-sensitive sodium transport (I(Na)) with a time constant of 18 min and half maximal inhibition constant of 1 microM. Analysis of amiloride analogue blocker-induced fluctuations in I(Na) showed linear rate-concentration plots with identical blocker on and off rates in control and aprotinin-inhibited conditions. Verification of open-block kinetics allowed for the use of a pulse protocol method (Helman, S.I., X. Liu, K. Baldwin, B.L. Blazer-Yost, and W.J. Els. 1998. Am. J. Physiol. 274:C947-C957) to study the same cells under different conditions as well as the reversibility of the aprotinin effect on single channel properties. Aprotinin caused reversible changes in all three single channel properties but only the change in the number of open channels was consistent with the inhibition of I(Na). A 50% decrease in I(Na) was accompanied by 50% increases in the single channel current and open probability but an 80% decrease in the number of open channels. Washout of aprotinin led to a time-dependent restoration of I(Na) as well as the single channel properties to the control, pre-aprotinin, values. We conclude that protease regulation of I(Na) is mediated by changes in the number of open channels in the apical membrane. The increase in the single channel current caused by protease inhibition can be explained by a hyperpolarization of the apical membrane potential as active Na(+) channels are retrieved. The paradoxical increase in channel open probability caused by protease inhibition will require further investigation but does suggest a potential compensatory regulatory mechanism to maintain I(Na) at some minimal threshold value.
Figures




References
-
- Adachi, M., K. Kitamura, T. Miyoshi, T. Narikiyo, K. Iwashita, N. Shiraishi, H. Nonoguchi, and K. Tomita. 2001. Activation of epithelial sodium channels by prostasin in Xenopus oocytes. J. Am. Soc. Nephrol. 12:1114–1121. - PubMed
-
- Baxendale-Cox, L.M., R.L. Duncan, X. Liu, K. Baldwin, W.J. Els, and S.I. Helman. 1997. Steroid hormone-dependent expression of blocker-sensitive ENaCs in apical membranes of A6 epithelia. Am. J. Physiol. 273:C1650–C1656. - PubMed

