Potent inhibition of local and disseminated tumor growth in immunocompetent mouse models by a bispecific antibody construct specific for Murine CD3
- PMID: 16187083
- PMCID: PMC11029878
- DOI: 10.1007/s00262-005-0082-x
Potent inhibition of local and disseminated tumor growth in immunocompetent mouse models by a bispecific antibody construct specific for Murine CD3
Abstract
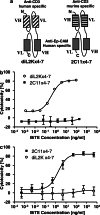
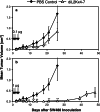
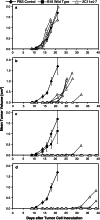
Bispecific single-chain antibody constructs specific for human CD3 have been extensively studied for antitumor activity in human xenograft models using severe combined immunodeficient mice supplemented with human T cells. High efficacy at low effector-to-target ratios, independence of T cell costimuli and a potent activation of previously unstimulated polyclonal T cells were identified as hallmarks of this class of bispecific antibodies. Here we studied a bispecific single-chain antibody construct (referred to as 'bispecific T cell engager', BiTE) in an immunocompetent mouse model. This was possible by the use of a murine CD3-specific BiTE, and a syngeneic melanoma cell line (B16F10) expressing the human Ep-CAM target. The murine CD3-specific BiTE, called 2C11x4-7 prevented in a dose-dependent fashion the outgrowth of subcutaneously growing B16/Ep-CAM tumors with daily i.v. injections of 5 or 50 microg BiTE which was most effective. Treatment with 2C11x4-7 was effective even when it was started 10 days after tumor cell inoculation but delayed treatments showed a reduction in the number of cured animals. 2C11x4-7 was also highly active in a lung tumor colony model. When treatment was started on the day of intravenous tumor cell injection, seven out of eight animals stayed free of lung tumors, and three out of eight animals when treatment was started on day 5. Our study shows that BiTEs also have a high antitumor activity in immunocompetent mice and that there is no obvious need for costimulation of T cells by secondary agents.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

