Vaccination of melanoma patients using dendritic cells loaded with an allogeneic tumor cell lysate
- PMID: 16187085
- PMCID: PMC11030805
- DOI: 10.1007/s00262-005-0078-6
Vaccination of melanoma patients using dendritic cells loaded with an allogeneic tumor cell lysate
Abstract
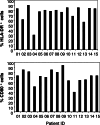
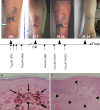
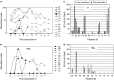
The aim of the present phase I/II study was to evaluate the safety, immune responses and clinical activity of a vaccine based on autologous dendritic cells (DC) loaded with an allogeneic tumor cell lysate in advanced melanoma patients. DC derived from monocytes were generated in serum-free medium containing GM-CSF and IL-13 according to Good Manufacturing Practices. Fifteen patients with metastatic melanoma (stage III or IV) received four subcutaneous, intradermal, and intranodal vaccinations of both DC loaded with tumor cell lysate and DC loaded with hepatitis B surface protein (HBs) and/or tetanus toxoid (TT). No grade 3 or 4 adverse events related to the vaccination were observed. Enhanced immunity to the allogeneic tumor cell lysate and to TAA-derived peptides were documented, as well as immune responses to HBs/TT antigens. Four out of nine patients who received the full treatment survived for more than 20 months. Two patients showed signs of clinical response and received 3 additional doses of vaccine: one patient showed regression of in-transit metastases leading to complete remission. Eighteen months later, the patient was still free of disease. The second patient experienced stabilization of lung metastases for approximately 10 months. Overall, our results show that vaccination with DC loaded with an allogeneic melanoma cell lysate was feasible in large-scale and well-tolerated in this group of advanced melanoma patients. Immune responses to tumor-related antigens documented in some treated patients support further investigations to optimize the vaccine formulation.
Figures


References
-
- Hartmann E, Wollenberg B, Rothenfusser S, Wagner M, Wellisch D, Mack B, Giese T, Gires O, Endres S, Hartmann G. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 2003;63:6478–6487. - PubMed
-
- Huang A, Gilmour JW, Imami N, Amjadi P, Henderson DC, Allen-Mersh TG. Increased serum transforming growth factor-beta1 in human colorectal cancer correlates with reduced circulating dendritic cells and increased colonic Langerhans cell infiltration. Clin Exp Immunol. 2003;134:270–278. doi: 10.1046/j.1365-2249.2003.02295.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

