Side population cells derived from adult human liver generate hepatocyte-like cells in vitro
- PMID: 16187169
- PMCID: PMC2676905
- DOI: 10.1007/s10620-005-2933-x
Side population cells derived from adult human liver generate hepatocyte-like cells in vitro
Abstract
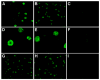
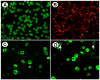
We sought to determine whether hepatic side population (SP) cells derived from adult human liver possess the potential of a novel candidate hepatic stem cell. Human cadaveric donor liver was subjected to collagenase perfusion and hepatocytes were separated from nonparenchymal cells by differential centrifugation. SP cells were isolated from the nonparenchymal portion after Hoechst 33342 staining. Since CD45 is a panleukocyte antigen, CD45-negative SP cells were separated from the vast majority of CD45-positive SP cells (90%), and hepatic growth medium was used to culture both groups. Both CD45-negative and CD45-positive hepatic SP cells generated colonies in the hepatic growth medium in 2-3 weeks. The colonies yielded large cells morphologically consistent with human hepatocytes, demonstrating granule-rich cytoplasm, dense, often double nuclei, and intracellular lipofuscin pigment. The cultured cells from both sources were positive for markers of human hepatocytes: HepPar, cytokeratin 8 (CK8), and human albumin. Reverse transcriptase-polymerase chain reaction (RT-PCR) performed on both groups demonstrated positivity for additional liver markers including human albumin, CK18, alpha-1 anti-trypsin, and the human cytochrome P450 enzyme CYP2B6. Double immunostaining (CD45 and HepPar) and RT-PCR confirmed that the hepatocyte-like cells derived from the CD45-negative SP cells acquired HepPar positivity but had no detectable CD45 antigen expression. In contrast, the cultured cells derived from the CD45-positive SP cells also acquired HepPar positivity, but only a minimal fraction expressed the CD45 antigen. We conclude that hepatic SP cells derived from the nonparenchymal portion of human liver are a potential source of human hepatocytes irrespective of their CD45 status, and further animal studies will be required to assess their regenerative potential.
Figures




References
-
- Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. - PubMed
-
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. - PubMed
-
- Forbes S, Vig P, Poulsom R, Thomas H, Alison M. Hepatic stem cells. J Pathol. 2002;197:510–518. - PubMed
-
- Oh SH, Hatch HM, Petersen BE. Hepatic oval ‘stem’ cell in liver regeneration. Semin Cell Dev Biol. 2002;13:405–409. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

