Semaphorin 4D activates the MAPK pathway downstream of plexin-B1
- PMID: 16187944
- PMCID: PMC1408676
- DOI: 10.1042/BJ20051123
Semaphorin 4D activates the MAPK pathway downstream of plexin-B1
Abstract
Semaphorins are a large family of transmembrane and secreted proteins that signal primarily through the receptor plexin. Semaphorins have been characterized in the nervous system as axon guidance cues; however, they have also been shown to control development of other cellular systems such as the vasculature and lungs. As the role of semaphorins outside of the nervous system has broadened, so has elucidation of the intracellular signalling pathways they initiate. Previously, we and others have shown that plexin-B1 activates RhoA through the binding and activation of RhoGEF (guanine nucleotide-exchange factor)/LARG (leukaemia-associated RhoGEF) in response to semaphorin 4D stimulation. In the present study, we show that semaphorin 4D activates the MAPK (mitogen-activated protein kinase) pathway. We have found that the mechanism of activation requires the C-terminus of plexin-B1 and the activation of RhoA.
Figures

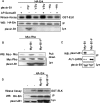

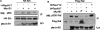

References
-
- Spriggs M. K. Shared resources between the neural and immune systems: semaphorins join the ranks. Curr. Opin. Immunol. 1999;11:387–391. - PubMed
-
- Christensen C. R., Klingelhofer J., Tarabykina S., Hulgaard E. F., Kramerov D., Lukanidin E. Transcription of a novel mouse semaphorin gene, M-semaH, correlates with the metastatic ability of mouse tumor cell lines. Cancer Res. 1998;58:1238–1244. - PubMed
-
- Behar O., Golden J. A., Mashimo H., Schoen F. J., Fishman M. C. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature (London) 1996;383:525–528. - PubMed
-
- Basile J. R., Barac A., Zhu T., Guan K. L., Gutkind J. S. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer Res. 2004;64:5212–5224. - PubMed
-
- Ito T., Kagoshima M., Sasaki Y., Li C., Udaka N., Kitsukawa T., Fujisawa H., Taniguchi M., Yagi T., Kitamura H., Goshima Y. Repulsive axon guidance molecule Sema3A inhibits branching morphogenesis of fetal mouse lung. Mech. Dev. 2000;97:35–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

