Anti-factor Xa kinetics after intravenous enoxaparin in patients undergoing percutaneous coronary intervention: a population model analysis
- PMID: 16187968
- PMCID: PMC1884830
- DOI: 10.1111/j.1365-2125.2005.02452.x
Anti-factor Xa kinetics after intravenous enoxaparin in patients undergoing percutaneous coronary intervention: a population model analysis
Erratum in
- Br J Clin Pharmacol. 2005 Oct;60(4):455
Abstract
Aim: Recent studies have suggested that intravenous (i.v.) enoxaparin could be used as antithrombotic therapy in patients ongoing percutaneous coronary intervention (PCI). However, anti-Xa pharmacokinetics following different i.v. dosing regimens is not clearly established.
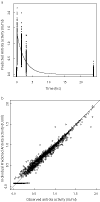
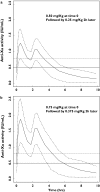
Methods: A population pharmacokinetic analysis was developed using anti-Xa activities measured in 546 patients who received a single 0.5 mg kg(-1) i.v. dose of enoxaparin immediately before PCI. Effects of higher doses (0.75 mg kg(-1) and 1 mg kg(-1)) and/or additional bolus after the initial administration were similarly simulated.
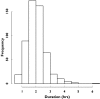
Results: Enoxaparin anti-Xa time profiles were best described by a one-compartment model with zero-order kinetics. Mean population parameters (intersubject variability, %) were CL 1.2 l h(-1) (33), V 2.9 l (30) and zero-order input 0.25 h (24). With a single bolus of 0.5 mg kg(-1), the totality of the patients reached an effective anticoagulation level (anti-Xa >0.5 IU ml(-1)) and only 2.5% reached levels above 1.5 IU ml(-1). Simulations showed that greater doses (0.75 mg kg(-1) and 1 mg kg(-1)) prolonged the duration of anticoagulation (3.4 and 4.1 h, respectively) compared with the 0.5 mg kg(-1) bolus (2.7 h) and markedly increased the proportion (48% and 79%, respectively) of patients with anti-Xa levels >1.5 IU ml(-1). For delayed and/or prolonged procedures, patients could be administered a second bolus of half the initial dose in a time interval between 90 min to 2 h after in order to maintain similar anticoagulation profile levels.
Conclusions: A single 0.5 mg kg(-1) i.v. dose of enoxaparin reached anticoagulation levels adequately and should be safer compared with greater doses for anticoagulation in patients undergoing an elective PCI. An additional second bolus could be proposed in patients with delayed or prolonged procedures.
Figures

References
-
- Braunwald E. Application of current guidelines to the management of unstable angina and non-ST-elevation myocardial infarction. Circulation. 2003;108(Suppl. III):III-28–III-37. 90161. - PubMed
-
- Popma JJ, Ohman EM, Weitz J, Lincoff AM, Harrington RA, Berger P. Antithrombotic therapy in patients undergoing PCI. Chest. 2001;119(Suppl.):321S–336S. - PubMed
-
- Holmes DRJ, Vlietstra RE, Smith HC, Vetrovec GW, Kent KM, Cowley MJ, Faxon DP, Gruentzig AR, Kelsey SF, Detre KM. Restenosis after percutaneous transluminal coronary angioplasty (PTCA): a report from the PTCA Registry of the National Heart, Lung, and Blood Institute. Am J Cardiol. 1984;53:77C–81C. - PubMed
-
- Guiteras Val P, Bourassa MG, David PR, Bonan R, Crepeau J, Dyrda I, Lesperance J. Restenosis after successful percutaneous transluminal coronary angioplasty: the Montreal Heart Institute experience. Am J Cardiol. 1987;60:50B–55B. - PubMed
-
- MacDonald RG, Barbieri E, Feldman RL, Pepine CJ. Angiographic morphology of restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1987;60:50–4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

