S-Naproxen and desmethylnaproxen glucuronidation by human liver microsomes and recombinant human UDP-glucuronosyltransferases (UGT): role of UGT2B7 in the elimination of naproxen
- PMID: 16187975
- PMCID: PMC1884820
- DOI: 10.1111/j.1365-2125.2005.02446.x
S-Naproxen and desmethylnaproxen glucuronidation by human liver microsomes and recombinant human UDP-glucuronosyltransferases (UGT): role of UGT2B7 in the elimination of naproxen
Abstract
Aims: To characterize the kinetics of S-naproxen ('naproxen') acyl glucuronidation and desmethylnaproxen acyl and phenolic glucuronidation by human liver microsomes and identify the human UGT isoform(s) catalysing these reactions.
Methods: Naproxen and desmethylnaproxen glucuronidation were investigated using microsomes from six and five livers, respectively. Human recombinant UGTs were screened for activity towards naproxen and desmethylnaproxen. Where significant activity was observed, kinetic parameters were determined. Naproxen and desmethylnaproxen glucuronides were measured by separate high-performance liquid chromatography methods.
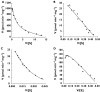
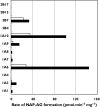
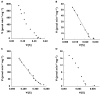
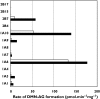
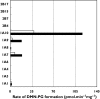
Results: Naproxen acyl glucuronidation by human liver microsomes followed biphasic kinetics. Mean apparent K(m) values (+/-SD, with 95% confidence interval in parentheses) for the high- and low-affinity components were 29 +/- 13 microm (16, 43) and 473 +/- 108 microm (359, 587), respectively. UGT 1A1, 1A3, 1A6, 1A7, 1A8, 1A9, 1A10 and 2B7 glucuronidated naproxen. UGT2B7 exhibited an apparent K(m) (72 microm) of the same order as the high-affinity human liver microsomal activity, which was inhibited by the UGT2B7 selective 'probe' fluconazole. Although data for desmethylnaproxen phenolic glucuronidation by human liver microsomes were generally adequately fitted to either the single- or two-enzyme Michaelis-Menten equation, model fitting was inconclusive for desmethylnaproxen acyl glucuronidation. UGT 1A1, 1A7, 1A9 and 1A10 catalysed both the phenolic and acyl glucuronidation of desmethylnaproxen, while UGT 1A3, 1A6 and 2B7 formed only the acyl glucuronide. Atypical glucuronidation kinetics were variably observed for naproxen and desmethylnaproxen glucuronidation by the recombinant UGTs.
Conclusion: UGT2B7 is responsible for human hepatic naproxen acyl glucuronidation, which is the primary elimination pathway for this drug.
Figures
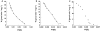
References
-
- Prescription cost analysis data. Drugs used in rheumatic diseases and gout. http://www.publications.doh.gov.uk/prescriptionstatistics/
-
- Upton RA, Buskin JN, Williams RL, Holford NHG, Riegelman S. Negligible excretion of unchanged ketoprofen, naproxen and probenecid in urine. J Pharm Sci. 1980;69:1254–7. - PubMed
-
- Runkel R, Chaplin M, Sevelius H, Ortega E, Segre E. Pharmacokinetics of naproxen overdoses. Clin Pharmacol Ther. 1976;24:269–77. - PubMed
-
- Miners JO, Coulter S, Tukey RH, Veronese ME, Birkett DJ. Cytochromes P450 1A2 and 2C9 are responsible for the human hepatic O-demethylation of R- and S-naproxen. Biochem Pharmacol. 1996;51:1003–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

