Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells
- PMID: 16188972
- PMCID: PMC1235841
- DOI: 10.1128/JVI.79.20.12692-12702.2005
Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells
Abstract
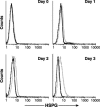
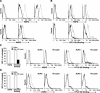
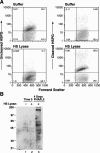
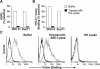
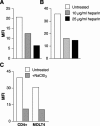
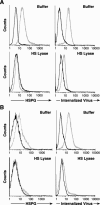
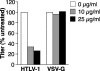
Heparan sulfate proteoglycans (HSPGs) are used by a number of viruses to facilitate entry into host cells. For the retrovirus human T-cell leukemia virus type 1 (HTLV-1), it has recently been reported that HSPGs are critical for efficient binding of soluble HTLV-1 SU and the entry of HTLV pseudotyped viruses into non-T cells. However, the primary in vivo targets of HTLV-1, CD4(+) T cells, have been reported to express low or undetectable levels of HSPGs. For this study, we reexamined the expression of HSPGs in CD4(+) T cells and examined their role in HTLV-1 attachment and entry. We observed that while quiescent primary CD4(+) T cells do not express detectable levels of HSPGs, HSPGs are expressed on primary CD4(+) T cells following immune activation. Enzymatic modification of HSPGs on the surfaces of either established CD4(+) T-cell lines or primary CD4(+) T cells dramatically reduced the binding of both soluble HTLV-1 SU and HTLV-1 virions. HSPGs also affected the efficiency of HTLV-1 entry, since blocking the interaction with HSPGs markedly reduced both the internalization of HTLV-1 virions and the titer of HTLV-1 pseudotyped viral infection in CD4(+) T cells. Thus, HSPGs play a critical role in the binding and entry of HTLV-1 into CD4(+) T cells.
Figures


References
-
- Altmeyer, R. 2004. Virus attachment and entry offer numerous targets for antiviral therapy. Curr. Pharm. Des. 10:3701-3712. - PubMed
-
- Argyris, E. G., E. Acheampong, G. Nunnari, M. Mukhtar, K. J. Williams, and R. J. Pomerantz. 2003. Human immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts. J. Virol. 77:12140-12151. - PMC - PubMed
-
- Barth, H., C. Schafer, M. I. Adah, F. Zhang, R. J. Linhardt, H. Toyoda, A. Kinoshita-Toyoda, T. Toida, T. H. Van Kuppevelt, E. Depla, F. Von Weizsacker, H. E. Blum, and T. F. Baumert. 2003. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J. Biol. Chem. 278:41003-41012. - PubMed
-
- Bernard, K. A., W. B. Klimstra, and R. E. Johnston. 2000. Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology 276:93-103. - PubMed
-
- Bernfield, M., M. Gotte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68:729-777. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

