Activation of the Jun N-terminal kinase pathway by friend spleen focus-forming virus and its role in the growth and survival of friend virus-induced erythroleukemia cells
- PMID: 16188978
- PMCID: PMC1235824
- DOI: 10.1128/JVI.79.20.12752-12762.2005
Activation of the Jun N-terminal kinase pathway by friend spleen focus-forming virus and its role in the growth and survival of friend virus-induced erythroleukemia cells
Abstract
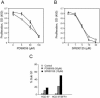
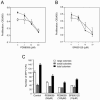
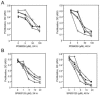
Members of the mitogen-activated protein kinase (MAPK) family, including Jun amino-terminal kinase (JNK) and extracellular signal-related kinase (ERK), play an important role in the proliferation of erythroid cells in response to erythropoietin (Epo). Erythroid cells infected with the Friend spleen focus-forming virus (SFFV) proliferate in the absence of Epo and show constitutive activation of Epo signal transduction pathways. We previously demonstrated that the ERK pathway was constitutively activated in Friend SFFV-infected erythroid cells, and in this study JNK is also shown to be constitutively activated. Pharmacological inhibitors of both the ERK and JNK pathways stopped the proliferation of primary erythroleukemic cells from Friend SFFV-infected mice, with little induction of apoptosis, and furthermore blocked their ability to form Epo-independent colonies. However, only the JNK inhibitor blocked the proliferation of erythroleukemia cell lines derived from these mice. The JNK inhibitor caused significant apoptosis in these cell lines as well as an increase in the fraction of cells in G(2)/M and undergoing endoreduplication. In contrast, the growth of erythroleukemia cell lines derived from Friend murine leukemia virus (MuLV)-infected mice was inhibited by both the MEK and JNK inhibitors. JNK is important for AP1 activity, and we found that JNK inhibitor treatment reduced AP1 DNA-binding activity in primary erythroleukemic splenocytes from Friend SFFV-infected mice and in erythroleukemia cell lines from Friend MuLV-infected mice but did not alter AP1 DNA binding in erythroleukemia cell lines from Friend SFFV-infected mice. These data suggest that JNK plays an important role in cell proliferation and/or the survival of erythroleukemia cells.
Figures





Similar articles
-
Both the polycythemia- and anemia-inducing strains of Friend spleen focus-forming virus induce constitutive activation of the Raf-1/mitogen-activated protein kinase signal transduction pathway.J Virol. 1998 Feb;72(2):919-25. doi: 10.1128/JVI.72.2.919-925.1998. J Virol. 1998. PMID: 9444983 Free PMC article.
-
Erythroleukaemia induction by the Friend spleen focus-forming virus.Baillieres Clin Haematol. 1995 Mar;8(1):225-47. doi: 10.1016/s0950-3536(05)80239-2. Baillieres Clin Haematol. 1995. PMID: 7663048 Review.
-
Erythroblast transformation by the friend spleen focus-forming virus is associated with a block in erythropoietin-induced STAT1 phosphorylation and DNA binding and correlates with high expression of the hematopoietic phosphatase SHP-1.J Virol. 2006 Jun;80(12):5678-85. doi: 10.1128/JVI.02651-05. J Virol. 2006. PMID: 16731906 Free PMC article.
-
Constitutive activation of Stat-related DNA-binding proteins in erythroid cells by the Friend spleen focus-forming virus.Leukemia. 1997 Apr;11 Suppl 3:251-4. Leukemia. 1997. PMID: 9209356
-
Deregulation of erythropoiesis by the Friend spleen focus-forming virus.Int J Biochem Cell Biol. 1999 Oct;31(10):1089-109. doi: 10.1016/s1357-2725(99)00074-6. Int J Biochem Cell Biol. 1999. PMID: 10582341 Review.
Cited by
-
Friend Spleen Focus-Forming Virus Activates the Tyrosine Kinase sf-Stk and the Transcription Factor PU.1 to Cause a Multi-Stage Erythroleukemia in Mice.Viruses. 2010 Oct;2(10):2235-2257. doi: 10.3390/v2102235. Epub 2010 Oct 11. Viruses. 2010. PMID: 21994618 Free PMC article.
-
JNK-mediated turnover and stabilization of the transcription factor p45/NF-E2 during differentiation of murine erythroleukemia cells.Proc Natl Acad Sci U S A. 2010 Jan 5;107(1):52-7. doi: 10.1073/pnas.0909153107. Epub 2009 Dec 4. Proc Natl Acad Sci U S A. 2010. PMID: 19966288 Free PMC article.
-
HIV-1 Nef inhibits lipopolysaccharide-induced IL-12p40 expression by inhibiting JNK-activated NFkappaB in human monocytic cells.J Biol Chem. 2009 Mar 20;284(12):7578-87. doi: 10.1074/jbc.M710013200. Epub 2008 Nov 19. J Biol Chem. 2009. Retraction in: J Biol Chem. 2012 Jan 2;287(1):804. doi: 10.1074/jbc.A111.710013. PMID: 19019824 Free PMC article. Retracted.
-
The role of tumor suppressor p15Ink4b in the regulation of hematopoietic progenitor cell fate.Blood Cancer J. 2013 Jan;3(1):e99. doi: 10.1038/bcj.2012.44. Epub 2013 Jan 4. Blood Cancer J. 2013. PMID: 23359317 Free PMC article.
-
Role of N-terminal sequences of the tyrosine kinase sf-Stk in transformation of rodent fibroblasts by variants of Friend spleen focus-forming virus.Int J Cancer. 2012 Sep 1;131(5):1083-94. doi: 10.1002/ijc.27330. Epub 2011 Dec 5. Int J Cancer. 2012. PMID: 22034044 Free PMC article.
References
-
- Ben-David, Y., and A. Bernstein. 1991. Friend virus-induced erythroleukemia and the multistage nature of cancer. Cell 66:831-834. - PubMed
-
- Ben-David, Y., E. G. Giddens, K. Letwin, and A. Bernstein. 1991. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Dev. 5:908-918. - PubMed
-
- Bennett, B. L., D. T. Sasaki, B. W. Murray, E. C. O'Leary, S. T. Sakata, W. Xu, J. C. Leisten, A. Motiwala, S. Pierce, Y. Satoh, S. S. Bhagwat, A. M. Manning, and D. W. Anderson. 2001. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 98:13681-13686. - PMC - PubMed
-
- Bergelson, S., U. Klingmuller, M. Socolovsky, J. Hsiao, and H. F. Lodish. 1998. Tyrosine residues within the intracellular domain of the erythropoietin receptor mediate activation of AP-1 transcription factors. J. Biol. Chem. 273:2396-2401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

