Low TRBP levels support an innate human immunodeficiency virus type 1 resistance in astrocytes by enhancing the PKR antiviral response
- PMID: 16188979
- PMCID: PMC1235869
- DOI: 10.1128/JVI.79.20.12763-12772.2005
Low TRBP levels support an innate human immunodeficiency virus type 1 resistance in astrocytes by enhancing the PKR antiviral response
Abstract
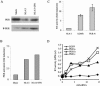
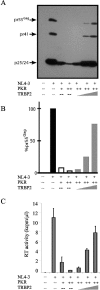
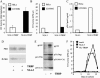
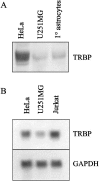
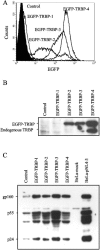
Acute human immunodeficiency virus type 1 (HIV-1) replication in astrocytes produces minimal new virus particles due, in part, to inefficient translation of viral structural proteins despite high levels of cytoplasmic viral mRNA. We found that a highly reactive double-stranded (ds) RNA-binding protein kinase (PKR) response in astrocytes underlies this inefficient translation of HIV-1 mRNA. The dsRNA elements made during acute replication of HIV-1 in astrocytes triggers PKR activation and the specific inhibition of HIV-1 protein translation. The heightened PKR response results from relatively low levels of the cellular antagonist of PKR, the TAR RNA binding protein (TRBP). Efficient HIV-1 production was restored in astrocytes by inhibiting the innate PKR response to HIV-1 dsRNA with dominant negative PKR mutants, or PKR knockdown by siRNA gene silencing. Increasing the expression of TRBP in astrocytes restored acute virus production to levels comparable to those observed in permissive cells. Therefore, the robust innate PKR antiviral response in astrocytes results from relatively low levels of TRBP expression and contributes to their restricted infection. Our findings highlight TRBP as a novel cellular target for therapeutic interventions to block productive HIV-1 replication in cells that are fully permissive for HIV-1 infection.
Figures

References
-
- Adelson, M. E., C. Martinand-Mari, K. T. Iacono, N. F. Muto, and R. J. Suhadolnik. 1999. Inhibition of human immunodeficiency virus (HIV-1) replication in SupT1 cells transduced with an HIV-1 LTR-driven PKR cDNA construct. Eur. J. Biochem. 264:806-815. - PubMed
-
- Bagasra, O., E. Lavi, L. Bobroski, K. Khalili, J. P. Pestaner, R. Tawadros, and R. J. Pomerantz. 1996. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS 10:573-585. - PubMed
-
- Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129-141. - PubMed
-
- Bannwarth, S., L. Talakoub, F. Letourneur, M. Duarte, D. F. Purcell, J. Hiscott, and A. Gatignol. 2001. Organization of the human tarbp2 gene reveals two promoters that are repressed in an astrocytic cell line. J. Biol. Chem. 276:48803-48813. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

