Hepatitis B virus large and middle glycoproteins are degraded by a proteasome pathway in glucosidase-inhibited cells but not in cells with functional glucosidase enzyme
- PMID: 16188993
- PMCID: PMC1235823
- DOI: 10.1128/JVI.79.20.12914-12920.2005
Hepatitis B virus large and middle glycoproteins are degraded by a proteasome pathway in glucosidase-inhibited cells but not in cells with functional glucosidase enzyme
Abstract
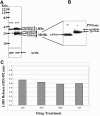
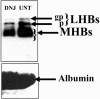
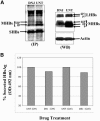
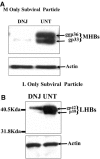
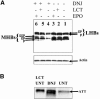
The secretion of hepatitis B virus (HBV) large (LHBs) and middle (MHBs) envelope polypeptides from tissue cultures requires proper protein folding and is prevented by inhibitors of the endoplasmic reticulum (ER) glucosidase. Using competitive inhibitors of the ER glucosidase, here it is shown that the amounts of glycosylated and unglycosylated forms of LHBs and MHBs proteins are all greatly reduced in tissue cultures producing HBV envelope glycoproteins. In contrast, the HBV small (SHBs) protein was not affected. The reduction in secretion of LHBs and MHBs proteins appears to be mediated by proteasomal degradation pathways, since it is prevented by either lactacystin or epoxomicin, two inhibitors of proteasomal degradation. Although there is no detectable proteasomal degradation of LHBs and MHBs in cells with functional glucosidase, the implications of the nearly quantitative sensitivity of glycosylated and unglycosylated forms of LHBs and MHBs proteins, with selective sparing of SHBs protein, in cells in which glucosidase is inhibited is surprising, and its implications are discussed.
Figures
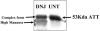
References
-
- Baumeister, W., J. Walz, F. Zuhl, and E. Seemuller. 1998. The proteasome: paradigm of a self-compartmentalizing protease. Cell 92:367-380. - PubMed
-
- Bergeron, J. J., M. B. Brenner, D. Y. Thomas, and D. B. Williams. 1994. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem. Sci. 19:124-128. - PubMed
-
- Block, T. M., X. Lu, A. S. Mehta, B. S. Blumberg, B. Tennant, M. Ebling, B. Korba, D. M. Lansky, G. S. Jacob, and R. A. Dwek. 1998. Treatment of chronic hepadnavirus infection in a woodchuck animal model with an inhibitor of protein folding and trafficking. Nat. Med. 4:610-614. - PubMed
-
- Block, T. M., and R. Jordan. 2002. Imino sugars as possible broad spectrum anti hepatitis virus agents: the alkovirs and glucovirs. Antiviral Chem. Chemother. 12:317-326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

