Induction and utilization of an ATM signaling pathway by polyomavirus
- PMID: 16189003
- PMCID: PMC1235815
- DOI: 10.1128/JVI.79.20.13007-13017.2005
Induction and utilization of an ATM signaling pathway by polyomavirus
Abstract
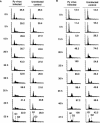
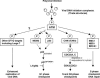
Progression from G(1) to S is essential for polyomavirus DNA replication and depends on the interaction of large T with the retinoblastoma gene product pRb. This virus-induced replication pathway is accompanied by p53 activation resembling a DNA damage response (12). We sought to determine whether this pathway depends in part on activation of the ATM (ataxia telangiectasia mutated) kinase and whether the virus gains advantages from this pathway beyond that of entry into S. We show that polyomavirus infection activates the S- and G(2)-phase checkpoints in primary as well as established mouse cells. Infected cells undergo a prolonged S phase compared to uninfected serum-stimulated cells and show no evidence of a G(2)-->M transition before lytic death ensues. Infection is accompanied by increases in ATM activity in vitro and in the level of ATM-S1981-P in vivo. The incubation of infected cells with caffeine, a known ATM inhibitor, did not block entry into S but reduced the rate of viral compared to cellular DNA synthesis. Importantly, caffeine lowered the yields of viral DNA an average of 3- to 6-fold and those of infectious virus by as much as 10-fold. Virus yields were 10-fold lower in ATM (-/-) p53(-/-) than in ATM(+/+) p53(-/-) mouse embryo fibroblasts, indicating a p53-independent role of ATM in productive infection. Replacement of the normal SMC1 (structural maintenance of chromosomes, or cohesin) protein, a critical ATM substrate in the DNA repair pathway, with its phosphorylation mutant SMC1(S957AS966A) also lowered virus yields by roughly 90%. We suggest that polyomavirus activates and utilizes a component(s) of an ATM pathway of DNA repair to prolong S phase and aid its own replication.
Figures





References
-
- Ahn, J. Y., J. K. Schwarz, H. Piwnica-Worms, and C. E. Canman. 2000. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 60:5934-5936. - PubMed
-
- Berkovich, E., and D. Ginsberg. 2003. ATM is a target for positive regulation by E2F-1. Oncogene 22:161-167. - PubMed
-
- Bracken, A. P., M. Ciro, A. Cocito, and K. Helin. 2004. E2F target genes: unraveling the biology. Trends Biochem. Sci. 29:409-417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

