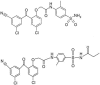
Antiviral activity of GW678248, a novel benzophenone nonnucleoside reverse transcriptase inhibitor
- PMID: 16189079
- PMCID: PMC1251513
- DOI: 10.1128/AAC.49.10.4046-4051.2005
Antiviral activity of GW678248, a novel benzophenone nonnucleoside reverse transcriptase inhibitor
Abstract
The compound GW678248 is a novel benzophenone nonnucleoside reverse transcriptase inhibitor (NNRTI). Preclinical assessment of GW678248 indicates that this compound potently inhibits wild-type (WT) and mutant human immunodeficiency virus type 1 (HIV-1) reverse transcriptase in biochemical assays, with 50% inhibitory concentrations (IC(50)s) between 0.8 and 6.8 nM. In HeLa CD4 MAGI cell culture virus replication assays, GW678248 has an IC(50) of < or =21 nM against HIV-1 isogenic strains with single or double mutations known to be associated with NNRTI resistance, including L100I, K101E, K103N, V106A/I/M, V108I, E138K, Y181C, Y188C, Y188L, G190A/E, P225H, and P236L and various combinations. An IC(50) of 86 nM was obtained with a mutant virus having V106I, E138K, and P236L mutations that resulted from serial passage of WT virus in the presence of GW678248. The presence of 45 mg/ml human serum albumin plus 1 mg/ml alpha-1 acid glycoprotein increased the IC(50) approximately sevenfold. Cytotoxicity studies with GW678248 indicate that the 50% cytotoxicity concentration is greater than the level of compound solubility and provides a selectivity index of >2,500-fold for WT, Y181C, or K103N HIV-1. This compound exhibits excellent preclinical antiviral properties and, as a prodrug designated GW695634, is being developed as a new generation of NNRTI for the treatment of HIV-1 in combination with other antiretroviral agents.
Figures
References
-
- Andries, K., H. Azijn, T. Thielemans, D. Ludovici, M. Kukla, J. Heeres, P. Janssen, B. De Corte, J. Vingerhoets, R. Pauwles, and M.-P. de Bethune. 2004. TMC125, a novel next-generation nonnucleoside reverse transcriptase inhibitor active against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 48:4680-4686. - PMC - PubMed
-
- Averett, D. R. 1989. Anti-HIV compound assessment by two novel high capacity assays. J. Virol. 23:263-276. - PubMed
-
- Bacheler, L., S. Jeffrey, G. Hanna, R. D'Aquila, L. Wallace, K. Logue, B. Cordova, K. Hertogs, B. Larder, R. Buckery, D. Baker, K. Gallagher, H. Scarnati, R. Tritch, and C. Rizzo. 2001. Genotypic correlates of phenotypic resistance to efavirenz in virus isolates from patients failing nonnucleoside reverse transcriptase inhibitor therapy. J. Virol. 75:4999-5008. - PMC - PubMed
-
- Bacheler, L. T., E. D. Anton, P. Kudish, D. Baker, J. Bunville, K. Krakowski, L. Bolling, M. Aujay, X. V. Wang, D. Ellis, M. F. Becker, A. L. Lasut, H. J. George, D. R. Spalding, G. Hollis, and K. Abremski. 2000. Human immunodeficiency virus type 1 mutation selected inpatients failing efavirenz combination therapy. Antimicrob. Agents Chemother. 44:2475-2484. - PMC - PubMed
-
- Boffito, M., D. J. Black, T. F. Blaschke, M. Rowland, R. J. Bertz, J. G. Gerber, and V. Miller. 2003. Protein binding in antiretroviral therapies. AIDS Res. Hum. Retrovir. 19:825-835. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials