Neuraminidase inhibitor-resistant influenza viruses may differ substantially in fitness and transmissibility
- PMID: 16189083
- PMCID: PMC1251536
- DOI: 10.1128/AAC.49.10.4075-4084.2005
Neuraminidase inhibitor-resistant influenza viruses may differ substantially in fitness and transmissibility
Abstract
Mutations of the conserved residues of influenza virus neuraminidase (NA) that are associated with NA inhibitor (NAI) resistance decrease the sialidase activity and/or stability of the NA, thus compromising viral fitness. In fact, clinically derived NAI-resistant variants with different NA mutations have shown different transmissibilities in ferrets (M. L. Herlocher, R. Truscon, S. Elias, H. Yen, N. A. Roberts, S. E. Ohmit, and A. S. Monto, J. Infect. Dis. 190:1627-1630, 2004). Molecular characterization of mutant viruses that have a homogeneous genetic background is required to determine the effect of single mutations at conserved NA residues. We generated recombinant viruses containing either the wild-type NA (RG WT virus) or a single amino acid change at NA residue 119 (RG E119V-NA virus) or 292 (RG R292K-NA virus) in the A/Wuhan/359/95 (H3N2) influenza virus background by reverse genetics. Both mutants showed decreased sensitivity to oseltamivir carboxylate, and the RG R292K-NA virus showed cross-resistance to zanamivir. We also observed differences between the two mutants in NA enzymatic activity and thermostability. The R292K mutation caused greater reduction of sialidase activity and thermostability than the E119V mutation. The NA defect caused by the R292K mutation was associated with compromised growth and transmissibility, whereas the growth and transmissibility of the RG E119V-NA virus were comparable to those of RG WT virus. Our results suggest that NAI-resistant influenza virus variants may differ substantially in fitness and transmissibility, depending on different levels of NA functional loss.
Figures


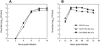


References
-
- Blick, T. J., A. Sahasrabudhe, M. McDonald, I. J. Owens, P. J. Morley, R. J. Fenton, and J. L. McKimm-Breschkin. 1998. The interaction of neuraminidase and hemagglutinin mutations in influenza virus in resistance to 4-guanidino-Neu5Ac2en. Virology 246:95-103. - PubMed
-
- Blick, T. J., T. Tiong, A. Sahasrabudhe, J. N. Varghese, P. M. Colman, G. J. Hart, R. C. Bethell, and J. L. McKimm-Breschkin. 1995. Generation and characterization of an influenza virus neuraminidase variant with decreased sensitivity to the neuraminidase-specific inhibitor 4-guanidino-Neu5Ac2en. Virology 214:475-484. - PubMed
-
- Carr, J., J. Ives, L. Kelly, R. Lambkin, J. Oxford, D. Mendel, L. Tai, and N. Roberts. 2002. Influenza virus carrying neuraminidase with reduced sensitivity to oseltamivir carboxylate has altered properties in vitro and is compromised for infectivity and replicative ability in vivo. Antiviral Res. 54:79-88. - PubMed
-
- Carr, J., J. Ives, N. Roberts, C. Y. Tai, M. Wang, M. Meng, D. Mendel, L. Kelly, R. Lambkin, and J. Oxford. 1999. An oseltamivir-treatment selected influenzaA/Wuhan/359/95 virus with an E119V mutation in the neuraminidase gene has reduced infectivity in vivo. In Second International Symposium for Influenza and Other Respiratory Viruses. The Macrae Group, New York, N.Y.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

