Transcriptional inhibitor of virulence factors in enteropathogenic Escherichia coli
- PMID: 16189086
- PMCID: PMC1251496
- DOI: 10.1128/AAC.49.10.4101-4109.2005
Transcriptional inhibitor of virulence factors in enteropathogenic Escherichia coli
Abstract
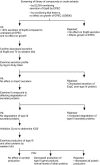
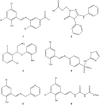
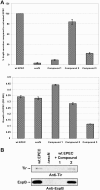
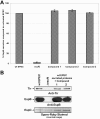
The type III secretion system (TTSS) is a key virulence mechanism of many important gram-negative bacterial pathogens. The TTSS is conserved among different bacterial pathogens, and mutations and deletions to the system significantly decrease virulence, making the TTSS an important potential therapeutic target. We have developed a high-throughput assay to search for inhibitors of the TTSS. We screened a commercial library of 20,000 small molecules for their ability to inhibit type III secretion by enteropathogenic Escherichia coli (EPEC). After discarding compounds that had no effect on secretion, inhibited bacterial growth, and/or caused degradation of EPEC-secreted proteins, the search was focused on a class of compounds that, while not direct inhibitors of type III secretion, inhibit expression of TTSS-related genes and other genes involved in virulence. This class of compounds does not affect bacterial viability or motility, indicating that it is not significantly affecting the expression of essential genes and is specific to virulence-associated genes. Transcriptional fusion assays confirmed that virulence-associated promoters were more sensitive to inhibition by this class of compounds. Overall, we have identified a class of compounds that can be used as a tool to probe the mechanism(s) that regulates virulence gene expression in EPEC.
Figures


References
-
- Bustamante, V. H., F. J. Santana, E. Calva, and J. L. Puente. 2001. Transcriptional regulation of type III secretion genes in enteropathogenic Escherichia coli: Ler antagonizes H-NS-dependent repression. Mol. Microbiol. 39:664-678. - PubMed
-
- Creasey, E. A., R. M. Delahay, S. Daniell, and G. Frankel. 2003. Yeast two-hybrid system survey of interactions between LEE-encoded proteins of enteropathogenic E. coli. Microbiology 148:2093-2106. - PubMed
-
- Deibel, C., S. Kramer, T. Chakraborty, and F. Ebel. 1998. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol. Microbiol. 28:463-474. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

