Multicenter study to determine antibody concentrations and assess the safety of administration of INH-A21, a donor-selected human Staphylococcal immune globulin, in low-birth-weight infants
- PMID: 16189088
- PMCID: PMC1251526
- DOI: 10.1128/AAC.49.10.4121-4127.2005
Multicenter study to determine antibody concentrations and assess the safety of administration of INH-A21, a donor-selected human Staphylococcal immune globulin, in low-birth-weight infants
Abstract
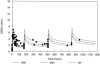
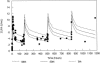
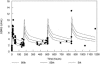
Nosocomial or late-onset sepsis is a common complication among premature infants, with a frequency inversely correlated with birth weight. Increased susceptibility to infection is due in part to an immature humoral (antibody-mediated) immune response. This study investigated the pharmacokinetics (PKs) and safety of a donor-selected specific intravenous immune globulin (IVIG) preparation, INH-A21 (Veronate), for prevention of sepsis in premature infants. Thirty-six infants weighing between 500 and 1,250 g during the first postnatal week were eligible to begin a series of up to four intravenous infusions of 500 or 750 mg/kg of body weight INH-A21. Blood samples were analyzed for antibodies against the Ser-Asp dipeptide repeat G (SdrG) and clumping factor A (ClfA) surface proteins of staphylococci. Sparse sampling and population PK analyses were performed to derive PK parameters. Following administration of the 500- and 750-mg/kg doses, the estimated average steady-state levels of anti-ClfA were 6.1 U/ml and 9.2 U/ml, respectively, and those of anti-SdrG were 5.2 U/ml and 7.7 U/ml, respectively. The elimination half-lives for anti-ClfA and anti-SdrG were 719 h and 701 h, respectively, and the clearances were 0.18 ml/h and 0.21 ml/h, respectively. In the final model, the values of the PK parameters were independent of gestational age. Both doses of INH-A21 were well tolerated, and the safety profile was similar to those of other IVIG preparations. These results suggest that a shorter dosing interval should be utilized between the first and second doses to achieve and maintain higher titers of anti-ClfA and anti-SdrG antibodies. Further studies examining INH-A21 for the prevention of late-onset sepsis in infants within the weight range studied are warranted.
Figures


References
-
- Anonymous. 1997. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics 99:93-99. - PubMed
-
- Baker, C. J., M. E. Melish, R. T. Hall, D. T. Casto, U. Vasan, L. B. Givner, et al. 1992. Intravenous immune globulin for the prevention of nosocomial infection in low-birth-weight neonates. N. Engl. J. Med. 327:213-219. - PubMed
-
- Barton, L., J. E. Hodgman, and Z. Pavlova. 1999. Causes of death in the extremely low birth weight infant. Pediatrics 103:446-451. - PubMed
-
- Capparelli, E. V., and J. D. Connor. 2002. Population pharmacokinetics of IVIG in premature infants using an endogenous IgG production model. Clin. Pharmacol. Ther. 71:P4.
-
- Fanaroff, A. A., M. Hack, and M. C. Walsh. 2003. The NICHD neonatal research network: changes in practice and outcomes during the first 15 years. Semin. Perinatol. 27:281-287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

