Pharmacodynamics of moxifloxacin against anaerobes studied in an in vitro pharmacokinetic model
- PMID: 16189103
- PMCID: PMC1251564
- DOI: 10.1128/AAC.49.10.4234-4239.2005
Pharmacodynamics of moxifloxacin against anaerobes studied in an in vitro pharmacokinetic model
Abstract
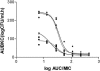
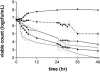
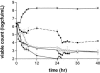
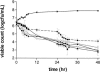
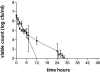
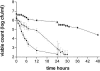
The antibacterial effects of moxifloxacin against Bacteroides fragilis, Clostridium perfringens, and gram-positive anaerobic cocci (GPAC) were studied in an in vitro pharmacokinetic model. Initially, a dose-ranging study with area under the concentration-time curve (AUC)/MIC ratios of 6.7 to 890 was used to investigate the effect of anaerobic conditions on the AUC/MIC antibacterial effect (ABE) relationship with Escherichia coli. The AUC/MIC ratios for 50% and 90% effects, using a log CFU drop at 24 h as the antibacterial effect measure, were 34 and 59, respectively, aerobic and 54 and 96, respectively, anaerobic. These values are not significantly different. Dose ranging at AUC/MIC ratios of 9 to 216 against the anaerobes indicated a differing AUC/MIC ABE pattern, and the AUC/MICs for 50% and 90% effects were lower: for B. fragilis, they were 10.5 and 25.7, respectively; for C. perfringens, they were 8.6 and 16.2; and for GPAC, they were 7.3 and 17.4. The maximum-effect log drops were as follows: for B. fragilis, -3.2 +/- 0.2 logs; for C. perfringens, -3.7 +/- 0.1 logs; and for GPAC, -2.5 +/- 0.1 logs. Although the anaerobes were not eradicated, there was no emergence of resistance. Comparison of the ABE of moxifloxacin to that of ertapenem against B. fragilis indicated that moxifloxacin was superior at 24 h and 48 h. In contrast, ertapenem was superior to moxifloxacin against GPAC at 24 h and 48 h and against C. perfringens at 48 h. Both drugs performed equivalently against C. perfringens at 24 h. Monte Carlo simulations using human serum AUC data and an AUC/MIC anaerobe target of 7.5 suggests a >90% target achievement at MICs of <2 mg/liter. This divides the B. fragilis wild-type MIC distribution. The pharmacodynamic properties of moxifloxacin against anaerobes are different than those against aerobic species. The clinical implications of these differences need further exploration.
Figures
Similar articles
-
Levofloxacin plus metronidazole administered once daily versus moxifloxacin monotherapy against a mixed infection of Escherichia coli and Bacteroides fragilis in an in vitro pharmacodynamic model.Antimicrob Agents Chemother. 2005 Feb;49(2):685-9. doi: 10.1128/AAC.49.2.685-689.2005. Antimicrob Agents Chemother. 2005. PMID: 15673752 Free PMC article.
-
Activity of moxifloxacin against Bacteroides fragilis and Escherichia coli in an in vitro pharmacokinetic/pharmacodynamic model employing pure and mixed cultures.J Med Microbiol. 2005 Aug;54(Pt 8):749-753. doi: 10.1099/jmm.0.45994-0. J Med Microbiol. 2005. PMID: 16014428
-
Moxifloxacin pharmacokinetics/pharmacodynamics and optimal dose and susceptibility breakpoint identification for treatment of disseminated Mycobacterium avium infection.Antimicrob Agents Chemother. 2010 Jun;54(6):2534-9. doi: 10.1128/AAC.01761-09. Epub 2010 Apr 12. Antimicrob Agents Chemother. 2010. PMID: 20385862 Free PMC article.
-
[Pharmacokinetic/pharmacodynamic analysis of antibiotic therapy in dentistry and stomatology].Enferm Infecc Microbiol Clin. 2005 Mar;23(3):116-21. doi: 10.1157/13072159. Enferm Infecc Microbiol Clin. 2005. PMID: 15757581 Review. Spanish.
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
Cited by
-
What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review.Clin Pharmacokinet. 2019 Nov;58(11):1407-1443. doi: 10.1007/s40262-019-00791-z. Clin Pharmacokinet. 2019. PMID: 31325141
-
Pharmacokinetics and tissue penetration of moxifloxacin in intervention therapy for intra-abdominal abscess.Clin Drug Investig. 2008;28(2):71-9. doi: 10.2165/00044011-200828020-00001. Clin Drug Investig. 2008. PMID: 18211115 Clinical Trial.
-
Moxifloxacin in the treatment of skin and skin structure infections.Ther Clin Risk Manag. 2006 Dec;2(4):417-34. doi: 10.2147/tcrm.2006.2.4.417. Ther Clin Risk Manag. 2006. PMID: 18360653 Free PMC article.
-
Moxifloxacinium chloride monohydrate.Acta Crystallogr Sect E Struct Rep Online. 2011 Oct 1;67(Pt 10):o2773-4. doi: 10.1107/S160053681103707X. Epub 2011 Sep 30. Acta Crystallogr Sect E Struct Rep Online. 2011. PMID: 22058817 Free PMC article.
-
Bacterial strain-to-strain variation in pharmacodynamic index magnitude, a hitherto unconsidered factor in establishing antibiotic clinical breakpoints.Antimicrob Agents Chemother. 2009 Dec;53(12):5181-4. doi: 10.1128/AAC.00118-09. Epub 2009 Oct 5. Antimicrob Agents Chemother. 2009. PMID: 19805569 Free PMC article.
References
-
- Andrews, J. 1999. Microbiological assays, p. 35-45. In D. S. Reeves, R. Wise, J. M. Andrews, and L. O. White (ed.), Clinical antimicrobial assays. Oxford University Press, Oxford, United Kingdom.
-
- British Society for Antimicrobial Chemotherapy Working Party. 1991. A guide to sensitivity testing. BSAC, Birmingham, United Kingdom.
-
- Brook, I., and J. D. Gillmore. 1993. In vitro susceptibility and in vivo efficacy of antimicrobials in the treatment of intraabdominal sepsis in mice. J. Antimicrob. Chemother. 31:393-401. - PubMed
-
- Dalhoff, A., and F.-J. Schmitz. 2003. In vitro antibacterial activity and pharmacodynamics of new quinolones. Eur. J. Clin. Microbiol. Infect. Dis. 22:203-221. - PubMed
-
- Donahue, P. E., D. L. Smith, A. E. Yellin, et al. 1998. Trovafloxacin in the treatment of intraabdominal infections: results of a double blind multicentre comparison with imipenem/cilistatin. Am. J. Surg. 197(Suppl. 6A):53-61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

