Dengue virus inoculation to human skin explants: an effective approach to assess in situ the early infection and the effects on cutaneous dendritic cells
- PMID: 16191104
- PMCID: PMC2517443
- DOI: 10.1111/j.0959-9673.2005.00445.x
Dengue virus inoculation to human skin explants: an effective approach to assess in situ the early infection and the effects on cutaneous dendritic cells
Abstract
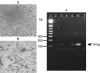
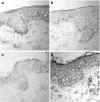
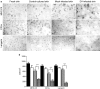
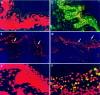
Although dengue virus (DV) enters through skin while mosquitoes feed, early contacts remain unexplored regarding the cutaneous viral fate and in situ immune responses. We addressed this by exposing healthy, non-cadaveric, freshly obtained human skin explants to a human DV2 isolate. We demonstrated negative-strand DV-RNA and non-structural protein-1, both suggestive of viral replication in skin. Although control, mock-infected and DV-infected explants showed less (MHC-CII(+)/CD1a(+)/Langerin+) Langerhans cells, deranged morphology and decreased frequency were more apparent in DV-infected explants. Whereas DV+ cells were infrequent in epidermis and completely absent in dermis, some areas of basal epidermis were clearly DV+, presumably keratinocytes, cells where TUNEL positivity revealed apoptosis. Unlike fresh, control and mock-infected skin, DV-infected explants expressed CD80 and CD83, indicative of dendritic cell (DC) activation and maturation, respectively. However, sequential sections indicated that these cells were not DV+, suggesting that activated/mature DCs capable of priming T cells, probably, were not infected. Alternatively, the occasionally infected epidermal DC might not have reached maturation. Interestingly, skin DV infection apparently uncouples the DC activation/maturation process from another crucial DC function, the subsequent migration into dermis. This was suggested, because upon cutaneous DV infection, the few emerging CD83+ (mature) DCs remained within the outer epidermis, while no dermal CD83+ DCs were observed. These paradoxical effects might represent unknown DV subversion strategies. This approach is relatively easy, quick (results in 48 h), economical for developing countries where dengue is re-emerging and advantageous to evaluate in situ viral biology, immunity and immunopathology and potential antiviral strategies.
Figures


Similar articles
-
Human skin Langerhans cells are targets of dengue virus infection.Nat Med. 2000 Jul;6(7):816-20. doi: 10.1038/77553. Nat Med. 2000. PMID: 10888933
-
[Study on dengue virus infection of human dendritic cells].Wei Sheng Wu Xue Bao. 2005 Aug;45(4):598-600. Wei Sheng Wu Xue Bao. 2005. PMID: 16245879 Chinese.
-
Herpes simplex virus infects skin gamma delta T cells before Langerhans cells and impedes migration of infected Langerhans cells by inducing apoptosis and blocking E-cadherin downregulation.J Immunol. 2010 Jul 1;185(1):477-87. doi: 10.4049/jimmunol.0904106. Epub 2010 Jun 2. J Immunol. 2010. PMID: 20519652
-
Innate immune responses to dengue virus.Arch Med Res. 2005 Sep-Oct;36(5):425-35. doi: 10.1016/j.arcmed.2005.04.007. Arch Med Res. 2005. PMID: 16099317 Review.
-
Monocytes, dendritic cells, and Langerhans cells in human immunodeficiency virus infection.Dermatol Clin. 1991 Jul;9(3):415-28. Dermatol Clin. 1991. PMID: 1873923 Review.
Cited by
-
Soluble mediators produced by the crosstalk between microvascular endothelial cells and dengue-infected primary dermal fibroblasts inhibit dengue virus replication and increase leukocyte transmigration.Immunol Res. 2016 Apr;64(2):392-403. doi: 10.1007/s12026-015-8675-8. Immunol Res. 2016. PMID: 26130295
-
Exploring plant-based dengue therapeutics: from laboratory to clinic.Trop Dis Travel Med Vaccines. 2024 Nov 15;10(1):23. doi: 10.1186/s40794-024-00232-1. Trop Dis Travel Med Vaccines. 2024. PMID: 39543749 Free PMC article.
-
A Mouse Model of Zika Virus Pathogenesis.Cell Host Microbe. 2016 May 11;19(5):720-30. doi: 10.1016/j.chom.2016.03.010. Epub 2016 Apr 5. Cell Host Microbe. 2016. PMID: 27066744 Free PMC article.
-
The battle between infection and host immune responses of dengue virus and its implication in dengue disease pathogenesis.ScientificWorldJournal. 2013;2013:843469. doi: 10.1155/2013/843469. Epub 2013 Feb 10. ScientificWorldJournal. 2013. PMID: 23476150 Free PMC article. Review.
-
Evasion of the human innate immune system by dengue virus.Immunol Res. 2012 Dec;54(1-3):152-9. doi: 10.1007/s12026-012-8334-2. Immunol Res. 2012. PMID: 22569913 Free PMC article. Review.
References
-
- Avirutnan P, Malasit P, Seliger B, Bhakdi S, Husmann M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J Immunol. 1998;161:6338–6346. - PubMed
-
- Bachmann MF, Zinkernagel RM, Oxenius A. Immune responses in the absence of costimulation: viruses know the trick. J Immunol. 1998;161:5791–5794. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

