Yeast kinetochore microtubule dynamics analyzed by high-resolution three-dimensional microscopy
- PMID: 16192284
- PMCID: PMC1366782
- DOI: 10.1529/biophysj.104.058461
Yeast kinetochore microtubule dynamics analyzed by high-resolution three-dimensional microscopy
Abstract
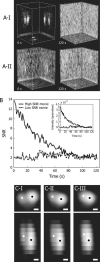
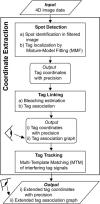
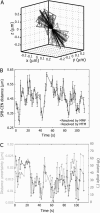
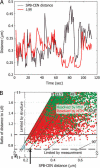
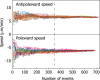
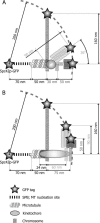
We have probed single kinetochore microtubule (k-MT) dynamics in budding yeast in the G1 phase of the cell cycle by automated tracking of a green fluorescent protein tag placed proximal to the centromere on chromosome IV and of a green fluorescent protein tag fused to the spindle pole body protein Spc42p. Our method reliably distinguishes between different dynamics in wild-type and mutant strains and under different experimental conditions. Using our methods we established that in budding yeast, unlike in metazoans, chromosomes make dynamic attachments to microtubules in G1. This makes it possible to interpret measurements of centromere tag dynamics as reflecting k-MT dynamics. We have examined the sensitivity of our assay by studying the effect of temperature, exposure to benomyl, and a tubulin mutation on k-MT dynamics. We have found that lowering the temperature and exposing cells to benomyl attenuate k-MT dynamics in a similar manner. We further observe that, in contrast to previous reports, the mutant tub2-150 forms k-MTs that depolymerize faster than wild type. Based on these findings, we propose high-resolution light microscopy of centromere dynamics in G1 yeast cells as a sensitive assay for the regulation of single k-MT dynamics.
Figures


References
-
- McAinsh, A. D., J. D. Tytell, and P. K. Sorger. 2003. Structure, function, and regulation of budding yeast kinetochores. Annu. Rev. Cell Dev. Biol. 19:519–539. - PubMed
-
- Mitchison, T., and M. Kirschner. 1984. Dynamic instability of microtubule growth. Nature. 312:237–242. - PubMed
-
- Kinoshita, K., I. Arnal, A. Desai, D. N. Drechsel, and A. A. Hyman. 2001. Reconstitution of physiological microtubule dynamics using purified components. Science. 294:1340–1343. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

