Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease
- PMID: 16192374
- PMCID: PMC6725603
- DOI: 10.1523/JNEUROSCI.2868-05.2005
Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease
Abstract
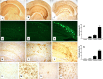
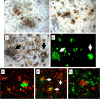
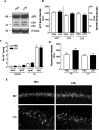
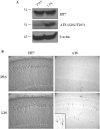
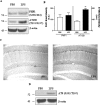
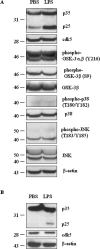
Inflammation is a critical component of the pathogenesis of Alzheimer's disease (AD). Although not an initiator of this disorder, inflammation nonetheless plays a pivotal role as a driving force that can modulate the neuropathology. Here, we characterized the time course of microglia activation in the brains of a transgenic model of AD (3xTg-AD) and discerned its relationship to the plaque and tangle pathology. We find that microglia became activated in a progressive and age-dependent manner, and this activation correlated with the onset of fibrillar amyloidbeta-peptide plaque accumulation and tau hyperphosphorylation. To determine whether microglial activation can exacerbate the pathology, we exposed young 3xTg-AD mice to lipopolysaccharide (LPS), a known inducer of CNS inflammation. Although amyloid precursor protein processing appeared unaffected, we find that LPS significantly induced tau hyperphosphorylation at specific sites that were mediated by the activation of cyclin-dependent kinase 5 (cdk5) through increased formation of the p25 fragment. We further show that administration of roscovitine, a selective and potent inhibitor of cdk5, markedly blocked the LPS-induced tau phosphorylation in the hippocampus. Therefore, this study clearly demonstrates that microglial activation exacerbates key neuropathological features such as tangle formation.
Figures


References
-
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, Nelson RB, McNeish JD (2000) Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci USA 97: 2910–2915. - PMC - PubMed
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, et al. (2000) Inflammation and Alzheimer's disease. Neurobiol Aging 21: 383–421. - PMC - PubMed
-
- Alvarez A, Toro R, Caceres A, Maccioni RB (1999) Inhibition of tau phosphorylating protein kinase cdk5 prevents beta-amyloid-induced neuronal death. FEBS Lett 459: 421–426. - PubMed
-
- Anthony JC, Breitner JC, Zandi PP, Meyer MR, Jurasova I, Norton MC, Stone SV (2000) Reduced prevalence of AD in users of NSAIDs and H2 receptor antagonists: the Cache County study. Neurology 54: 2066–2071. - PubMed
-
- Apelt J, Schliebs R (2001) Beta-amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res 894: 21–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
