Calcium/calmodulin-dependent protein kinase II alters structural plasticity and cytoskeletal dynamics in Drosophila
- PMID: 16192377
- PMCID: PMC6725600
- DOI: 10.1523/JNEUROSCI.2005-05.2005
Calcium/calmodulin-dependent protein kinase II alters structural plasticity and cytoskeletal dynamics in Drosophila
Abstract
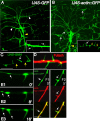
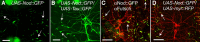
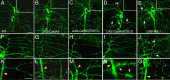
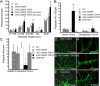
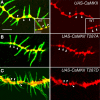
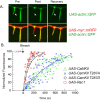
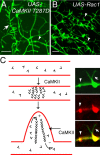
Drosophila dendritic arborization (da) neurons contain subclasses of neurons with distinct dendritic morphologies. We investigated calcium/calmodulin-dependent protein kinase II (CaMKII) regulation of dendritic structure and dynamics in vivo using optically transparent Drosophila larvae. CaMKII increases the dynamic nature and formation of dendritic filopodia throughout larval development but only affects neurons that normally contain dendritic filopodia. In parallel, we examined the effects of Rac1 activity on dendritic structure to explore signaling specificity. In contrast to CaMKII activity, Rac1 does not alter filopodia stability but instead causes de novo filopodia formation on all da neurons. Although both mediators increase cytoskeletal turnover, measured by fluorescence recovery after photobleaching experiments, only CaMKII increases the dynamic nature of dendritic filopodia. CaMKII signaling thus appears to use mechanisms and machinery distinct from Rac1 signaling. This study illustrates a molecular means of uncoupling cytoskeletal regulation from morphological regulation. Our results suggest that Drosophila dendritic filopodia may share some cytoskeletal regulatory mechanisms with mammalian dendritic filopodia. Furthermore, general dendrite cytoskeletal compartmentalization is conserved in multipolar neurons.
Figures
References
-
- Ainsley JA, Pettus JM, Bosenko D, Gerstein CE, Zinkevich N, Anderson MG, Adams CM, Welsh MJ, Johnson WA (2003) Enhanced locomotion caused by loss of the Drosophila DEG/ENaC protein Pickpocket1. Curr Biol 13: 1557–1563. - PubMed
-
- Bodmer R, Jan YN (1987) Morphological differentiation of the embryonic peripheral neurons in Drosophila. Rouxs Arch Dev Biol 196: 69–77. - PubMed
-
- Brand AH (1995) GFP in Drosophila. Trends Genet 11: 324–325. - PubMed
-
- Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
