Myosin light chain kinase is involved in lipopolysaccharide-induced disruption of colonic epithelial barrier and bacterial translocation in rats
- PMID: 16192642
- PMCID: PMC1603678
- DOI: 10.1016/S0002-9440(10)61196-0
Myosin light chain kinase is involved in lipopolysaccharide-induced disruption of colonic epithelial barrier and bacterial translocation in rats
Abstract
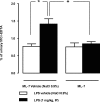
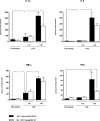
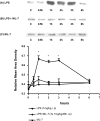
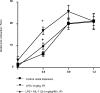
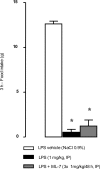
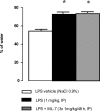
Sepsis is associated with bacterial translocation (BT) and changes in colonic paracellular permeability (CPP), but the link between these effects is unknown. The present study aimed to identify whether changes in CPP after lipopolysaccharide (LPS) administration triggers BT, colonic inflammation, visceral pain, and sickness behavior and to evaluate the role of myosin light chain kinase (MLCK) in colonocyte cytoskeleton contraction. Rats received the MLCK inhibitor ML-7 alone or combined with LPS. CPP was measured for 6 hours after administration. Visceral pain, food intake, BT, electron microscopy of tight junctions of colonocytes, cytokine levels, and Western blotting of phosphorylated MLC from colonic mucosa were assessed in a time range of 0 to 3 hours after treatment. Sepsis increased CPP at 0 to 6 hours after LPS and associated with tight junction morphological changes, increased MLC phosphorylation, and mucosal release of proinflammatory cytokines. Massive BT, visceral hyperalgesia, and reduced food intake were also observed. Addition of ML-7 prevented all LPS-induced effects, except for changes in food intake. In conclusion, LPS-mediated effects on CPP include gut inflammation, BT, and visceral hyperalgesia. Inhibition of MLCK-dependent colonocyte cytoskeleton contraction by ML-7 prevents the LPS-induced alterations of CPP and its subsequent effects.
Figures

Similar articles
-
Vasoactive intestinal peptide ameliorates intestinal barrier disruption associated with Citrobacter rodentium-induced colitis.Am J Physiol Gastrointest Liver Physiol. 2009 Oct;297(4):G735-50. doi: 10.1152/ajpgi.90551.2008. Epub 2009 Aug 6. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19661153
-
Mechanism of extracellular calcium regulation of intestinal epithelial tight junction permeability: role of cytoskeletal involvement.Microsc Res Tech. 2000 Oct 15;51(2):156-68. doi: 10.1002/1097-0029(20001015)51:2<156::AID-JEMT7>3.0.CO;2-J. Microsc Res Tech. 2000. PMID: 11054866
-
LPS-induced lung inflammation is linked to increased epithelial permeability: role of MLCK.Eur Respir J. 2005 May;25(5):789-96. doi: 10.1183/09031936.05.00064704. Eur Respir J. 2005. PMID: 15863634
-
The Regulation of Intestinal Mucosal Barrier by Myosin Light Chain Kinase/Rho Kinases.Int J Mol Sci. 2020 May 18;21(10):3550. doi: 10.3390/ijms21103550. Int J Mol Sci. 2020. PMID: 32443411 Free PMC article. Review.
-
Myosin light chain kinase: pulling the strings of epithelial tight junction function.Ann N Y Acad Sci. 2012 Jul;1258(1):34-42. doi: 10.1111/j.1749-6632.2012.06526.x. Ann N Y Acad Sci. 2012. PMID: 22731713 Free PMC article. Review.
Cited by
-
Effect of Lianshu preparation on lipopolysaccharide-induced diarrhea in rats.World J Gastroenterol. 2009 Apr 28;15(16):2009-15. doi: 10.3748/wjg.15.2009. World J Gastroenterol. 2009. PMID: 19399935 Free PMC article.
-
Discovery of BVDU as a promising Drug for autoimmune diseases Therapy by Dendritic-cell-based functional screening.Sci Rep. 2017 Mar 8;7:43820. doi: 10.1038/srep43820. Sci Rep. 2017. PMID: 28272439 Free PMC article.
-
Lipopolysaccharide-Induced Increase in Intestinal Epithelial Tight Permeability Is Mediated by Toll-Like Receptor 4/Myeloid Differentiation Primary Response 88 (MyD88) Activation of Myosin Light Chain Kinase Expression.Am J Pathol. 2017 Dec;187(12):2698-2710. doi: 10.1016/j.ajpath.2017.08.005. Am J Pathol. 2017. PMID: 29157665 Free PMC article.
-
The Gut-Brain Axis in Autism Spectrum Disorder: A Focus on the Metalloproteases ADAM10 and ADAM17.Int J Mol Sci. 2020 Dec 24;22(1):118. doi: 10.3390/ijms22010118. Int J Mol Sci. 2020. PMID: 33374371 Free PMC article. Review.
-
GYY4137, as a slow-releasing H2S donor, ameliorates sodium deoxycholate-induced chronic intestinal barrier injury and gut microbiota dysbiosis.Front Pharmacol. 2024 Oct 22;15:1476407. doi: 10.3389/fphar.2024.1476407. eCollection 2024. Front Pharmacol. 2024. PMID: 39508040 Free PMC article.
References
-
- O’Dwer ST, Michie HR, Ziegler TR, Revhaug A, Smith RJ, Wilmore DW. A single dose of endotoxin increases intestinal permeability in healthy humans. Arch Surg. 1988;123:1459–1464. - PubMed
-
- Wang Q, Pantzar N, Jeppsson B, Westrom BR, Karlsson BW. Increased intestinal marker absorption due to regional permeability changes and decreased intestinal transit during sepsis in rat. Scand J Gastroenterol. 1994;29:1001–1008. - PubMed
-
- Naaber P, Smidt I, Tamme K, Liigant A, Tapfer H, Mikelsaar M, Talvik R. Translocation of indigenous microflora in an experimental model of sepsis. J Med Microbiol. 2000;49:431–439. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

