Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations
- PMID: 16193063
- PMCID: PMC1276711
- DOI: 10.1038/sj.emboj.7600828
Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations
Abstract
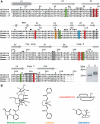
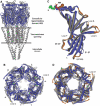
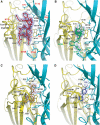
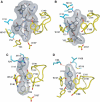
Upon ligand binding at the subunit interfaces, the extracellular domain of the nicotinic acetylcholine receptor undergoes conformational changes, and agonist binding allosterically triggers opening of the ion channel. The soluble acetylcholine-binding protein (AChBP) from snail has been shown to be a structural and functional surrogate of the ligand-binding domain (LBD) of the receptor. Yet, individual AChBP species display disparate affinities for nicotinic ligands. The crystal structure of AChBP from Aplysia californica in the apo form reveals a more open loop C and distinctive positions for other surface loops, compared with previous structures. Analysis of Aplysia AChBP complexes with nicotinic ligands shows that loop C, which does not significantly change conformation upon binding of the antagonist, methyllycaconitine, further opens to accommodate the peptidic antagonist, alpha-conotoxin ImI, but wraps around the agonists lobeline and epibatidine. The structures also reveal extended and nonoverlapping interaction surfaces for the two antagonists, outside the binding loci for agonists. This comprehensive set of structures reflects a dynamic template for delineating further conformational changes of the LBD of the nicotinic receptor.
Figures

References
-
- Badio B, Daly JW (1994) Epibatidine, a potent analgetic and nicotinic agonist. Mol Pharmacol 45: 563–569 - PubMed
-
- Bergmeier SC, Ismail KA, Arason KM, McKay S, Bryant DL, McKay DB (2004) Structure activity studies of ring E analogues of methyllycaconitine. Part 2: Synthesis of antagonists to the α3β4 nicotinic acetylcholine receptors through modifications to the ester. Bioorg Med Chem Lett 14: 3739–3742 - PubMed
-
- Bouzat C, Gumilar F, Spitzmaul G, Wang HL, Rayes D, Hansen SB, Taylor P, Sine SM (2004) Coupling of agonist binding to channel gating in an ACh-binding protein linked to an ion channel. Nature 430: 896–900 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

