Sequence variation in ligand binding sites in proteins
- PMID: 16194281
- PMCID: PMC1261162
- DOI: 10.1186/1471-2105-6-240
Sequence variation in ligand binding sites in proteins
Abstract
Background: The recent explosion in the availability of complete genome sequences has led to the cataloging of tens of thousands of new proteins and putative proteins. Many of these proteins can be structurally or functionally categorized from sequence conservation alone. In contrast, little attention has been given to the meaning of poorly-conserved sites in families of proteins, which are typically assumed to be of little structural or functional importance.
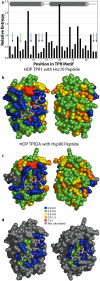
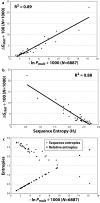
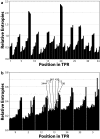
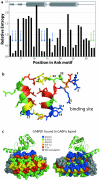
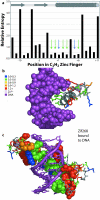
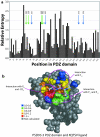
Results: Recently, using statistical free energy analysis of tetratricopeptide repeat (TPR) domains, we observed that positions in contact with peptide ligands are more variable than surface positions in general. Here we show that statistical analysis of TPRs, ankyrin repeats, Cys2His2 zinc fingers and PDZ domains accurately identifies specificity-determining positions by their sequence variation. Sequence variation is measured as deviation from a neutral reference state, and we present probabilistic and information theory formalisms that improve upon recently suggested methods such as statistical free energies and sequence entropies.
Conclusion: Sequence variation has been used to identify functionally-important residues in four selected protein families. With TPRs and ankyrin repeats, protein families that bind highly diverse ligands, the effect is so pronounced that sequence "hypervariation" alone can be used to predict ligand binding sites.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

