Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow
- PMID: 16195235
- PMCID: PMC1395485
- DOI: 10.1074/jbc.M504672200
Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow
Abstract
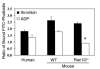
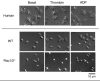
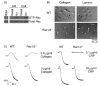
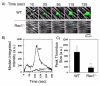
The role of Rac family proteins in platelet spreading on matrix proteins under static and flow conditions has been investigated by using Rac-deficient platelets. Murine platelets form filopodia and undergo limited spreading on fibrinogen independent of Rac1 and Rac2. In the presence of thrombin, marked lamellipodia formation is observed on fibrinogen, which is abrogated in the absence of Rac1. However, Rac1 is not required for thrombin-induced aggregation or elevation of F-actin levels. Formation of lamellipodia on collagen and laminin is also Rac1-dependent. Analysis of platelet adhesion dynamics on collagen under flow conditions in vitro revealed that Rac1 is required for platelet aggregate stability at arterial rates of shear, as evidenced by a dramatic increase in platelet embolization. Furthermore, studies employing intravital microscopy demonstrated that Rac1 plays a critical role in the development of stable thrombi at sites of vascular injury in vivo. Thus, our data demonstrated that Rac1 is essential for lamellipodia formation in platelets and indicated that Rac1 is required for aggregate integrity leading to thrombus formation under physiologically relevant levels of shear both in vitro and in vivo.
Figures






References
-
- Haataja L, Groffen J, Heisterkamp N. J. Biol. Chem. 1997;272:20384–20388. - PubMed
-
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. Cell. 1992;70:401–410. - PubMed
-
- Ridley AJ. Trends Cell Biol. 2001;11:471–477. - PubMed
-
- Burridge K, Wennerberg K. Cell. 2004;116:167–179. - PubMed
-
- Hartwig JH, Bokoch GM, Carpenter CL, Janmey PA, Taylor LA, Toker A, Stossel TP. Cell. 1995;82:643–653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

