Randomised controlled trials in Africa of HIV and AIDS: descriptive study and spatial distribution
- PMID: 16195291
- PMCID: PMC1239977
- DOI: 10.1136/bmj.331.7519.742
Randomised controlled trials in Africa of HIV and AIDS: descriptive study and spatial distribution
Abstract
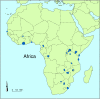
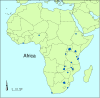
Objectives: To identify and describe randomised controlled trials on HIV and AIDS conducted in Africa and to map their spatial distribution using exact geographic coordinates.
Design: Construction and analysis of a database of trials conducted wholly or partly in Africa and reported before 2004.
Data sources: CENTRAL, Medline, Embase, and LILACS.
Results: Our comprehensive search yielded 284 distinct records that were potentially eligible for inclusion in the database. Of these, 150 articles reported on 77 eligible trials published or reported from 1987 to 2003. Seven trials were identified exclusively from the CENTRAL database. Trials were conducted in 18 of 48 countries in sub-Saharan Africa. None were conducted in north Africa. Only 19 had a principal investigator located in an African country. Forty two trials assessed prevention and 35 assessed treatment. Most studies were funded by government agencies outside Africa (n = 43), with the pharmaceutical industry providing partial support to 16 of these. The pharmaceutical industry provided full or partial support to a further 18 trials. Only 43 trials reported conducting a power calculation for determining sample size. There was no mention of ethical approval or informed consent in 19 and 17 trials, respectively.
Conclusion: The relatively small number of HIV/AIDS trials conducted in Africa is not commensurate with the burden of disease. Geographical mapping as an adjunct to prospective trial registration is a useful tool for researchers and decision makers to track existing and future trials.
Figures

References
-
- Schulz KF, Grimes DA. Generation of allocation sequences in randomised trials: chance, not choice. Lancet 2002;359: 515-9. - PubMed
-
- UNAIDS. 2004 report on the global AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS, 2004.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
