Signal transduction pathways and gating mechanisms of native TRP-like cation channels in vascular myocytes
- PMID: 16195316
- PMCID: PMC1464290
- DOI: 10.1113/jphysiol.2005.096875
Signal transduction pathways and gating mechanisms of native TRP-like cation channels in vascular myocytes
Abstract
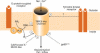
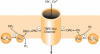
Activation of Ca2+-permeable non-selective cation channels produces an increase in excitability of vascular smooth muscle cells which has an important role in vasoconstriction. These channels are activated by various physiological stimuli including vasoconstrictor agents such as noradrenaline, depletion of internal Ca2+ stores and cell stretching. In addition cation channels have been shown to be constitutively active and these channels are thought to contribute to resting membrane conductance and basal Ca2+ influx in vascular myocytes. Recent evidence has suggested that transient receptor potential (TRP) proteins represent strong candidates for these channels in the vasculature. This review discusses proposed signal transduction pathways and gating mechanisms which link physiological stimuli to opening of cation channels in vascular myocytes. It is apparent that G-protein-coupled pathways linked to stimulation of phospholipase activity have a profound effect on regulating channel activity and that generation of diacylglycerol (DAG) is a central event in these signalling cascades with this triglyceride having a pivotal role in gating cation channels via both PKC-independent and -dependent mechanisms. Moreover phosphorylation processes produced by stimulation of protein kinases have been proposed to have an important role in regulating cation channel activity.
Figures

References
-
- Ahmmed GU, Mehta D, Vogel S, Holinstat M, Paria BC, Tiruppathi C, Malik AB. Protein kinase Cα phosphorylates transient receptor potential channel-1 (TRPC1) and regulates store-operated Ca2+ entry. Role in signalling increased endothelial permeability. J Biol Chem. 2004;279:20941–20949. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

