Temperature-modulated alternative splicing and promoter use in the Circadian clock gene frequency
- PMID: 16195340
- PMCID: PMC1289402
- DOI: 10.1091/mbc.e05-08-0756
Temperature-modulated alternative splicing and promoter use in the Circadian clock gene frequency
Abstract
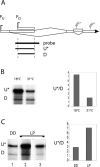
The expression of FREQUENCY, a central component of the circadian clock in Neurospora crassa, shows daily cycles that are exquisitely sensitive to the environment. Two forms of FRQ that differ in length by 99 amino acids, LFRQ and SFRQ, are synthesized from alternative initiation codons and the change in their ratio as a function of temperature contributes to robust rhythmicity across a range of temperatures. We have found frq expression to be surprisingly complex, despite our earlier prediction of a simple transcription unit based on limited cDNA sequencing. Two distinct environmentally regulated major promoters drive primary transcripts whose environmentally influenced alternative splicing gives rise to six different major mRNA species as well as minor forms. Temperature-sensitive alternative splicing determines AUG choice and, as a consequence, the ratio of LFRQ to SFRQ. Four of the six upstream ORFs are spliced out of the vast majority of frq mRNA species. Alternative splice site choice in the 5' UTR and relative use of two major promoters are also influenced by temperature, and the two promoters are differentially regulated by light. Evolutionary comparisons with the Sordariaceae reveal conservation of 5' UTR sequences, as well as significant conservation of the alternative splicing events, supporting their relevance to proper regulation of clock function.
Figures





References
-
- Aronson, B. D., Johnson, K. A., Loros, J. J., and Dunlap, J. C. (1994b). Negative feedback defining a circadian clock: autoregulation of the clock gene frequency. Science 263, 1578–1584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

