VirA and VirG activate the Ti plasmid repABC operon, elevating plasmid copy number in response to wound-released chemical signals
- PMID: 16195384
- PMCID: PMC1253548
- DOI: 10.1073/pnas.0503458102
VirA and VirG activate the Ti plasmid repABC operon, elevating plasmid copy number in response to wound-released chemical signals
Abstract
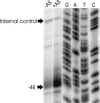
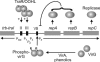
The vir genes of Agrobacterium tumefaciens tumor-inducing (Ti) plasmids direct the transfer of oncogenic portion of the Ti (tumor-inducing) plasmid that is transferred to plant cells (T-DNA) into plant cells and are coordinately induced by plant-released phenolic chemical signals. We have used DNA microarrays, representing all genes of the octopine- and nopaline-type Ti plasmids, to identify all Ti-plasmid-encoded genes in the vir regulons of both plasmids. Acetosyringone (AS) induced the expression of all known members of the vir regulons, as well as a small number of additional genes. Unexpectedly, AS also caused a modest induction of virtually every Ti plasmid gene. This suggested that the copy number of the Ti plasmid might increase in response to AS, a hypothesis confirmed by DNA dot blotting. VirA and VirG were the only Vir proteins required for this copy number increase. Promoter resections and primer extension analysis of the repABC promoter region showed that expression of the promoter closest to repA (promoter P4) was induced by AS. We also identified a sequence resembling a consensus VirG-binding motif approximately 70 nucleotides upstream from the P4 transcription start site. Mutating this sequence blocked the AS-induced copy number increase of a RepABC-dependent miniplasmid, indicating that phospho-VirG increases copy number solely by enhancing repABC expression.
Figures

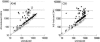





Similar articles
-
The RepA and RepB autorepressors and TraR play opposing roles in the regulation of a Ti plasmid repABC operon.Mol Microbiol. 2003 Jul;49(2):441-55. doi: 10.1046/j.1365-2958.2003.03560.x. Mol Microbiol. 2003. PMID: 12828641
-
A LuxR-type regulator from Agrobacterium tumefaciens elevates Ti plasmid copy number by activating transcription of plasmid replication genes.Mol Microbiol. 2003 May;48(4):1059-73. doi: 10.1046/j.1365-2958.2003.03488.x. Mol Microbiol. 2003. PMID: 12753196
-
RepB protein of an Agrobacterium tumefaciens Ti plasmid binds to two adjacent sites between repA and repB for plasmid partitioning and autorepression.Mol Microbiol. 2005 Nov;58(4):1114-29. doi: 10.1111/j.1365-2958.2005.04886.x. Mol Microbiol. 2005. PMID: 16262794
-
Cell-cell signaling and the Agrobacterium tumefaciens Ti plasmid copy number fluctuations.Plasmid. 2008 Sep;60(2):89-107. doi: 10.1016/j.plasmid.2008.05.003. Epub 2008 Aug 12. Plasmid. 2008. PMID: 18664372 Review.
-
Agrobacterium virulence gene induction.Methods Mol Biol. 2006;343:77-84. doi: 10.1385/1-59745-130-4:77. Methods Mol Biol. 2006. PMID: 16988335 Review.
Cited by
-
The ABCs of plasmid replication and segregation.Nat Rev Microbiol. 2012 Nov;10(11):755-65. doi: 10.1038/nrmicro2882. Nat Rev Microbiol. 2012. PMID: 23070556 Review.
-
Enhanced production of single copy backbone-free transgenic plants in multiple crop species using binary vectors with a pRi replication origin in Agrobacterium tumefaciens.Transgenic Res. 2011 Aug;20(4):773-86. doi: 10.1007/s11248-010-9458-6. Epub 2010 Nov 2. Transgenic Res. 2011. PMID: 21042934
-
Agrobacterium tumefaciens: a Transformative Agent for Fundamental Insights into Host-Microbe Interactions, Genome Biology, Chemical Signaling, and Cell Biology.J Bacteriol. 2023 Apr 25;205(4):e0000523. doi: 10.1128/jb.00005-23. Epub 2023 Mar 9. J Bacteriol. 2023. PMID: 36892285 Free PMC article. Review.
-
Ecological dynamics and complex interactions of Agrobacterium megaplasmids.Front Plant Sci. 2014 Nov 14;5:635. doi: 10.3389/fpls.2014.00635. eCollection 2014. Front Plant Sci. 2014. PMID: 25452760 Free PMC article. Review.
-
Effect of Agrobacterium strain and plasmid copy number on transformation frequency, event quality and usable event quality in an elite maize cultivar.Plant Cell Rep. 2015 May;34(5):745-54. doi: 10.1007/s00299-014-1734-0. Epub 2015 Jan 6. Plant Cell Rep. 2015. PMID: 25558819
References
-
- Johnson, T. M. & Das., A. (1998) in The Rhizobeaceae: Molecular Biology of Model Plant-Associated Bacteria, eds. Spaink, H. P., Kondorosi, A. & Hooykaas, P. J. J. (Kluwer, Dordrecht, The Netherlands), pp. 265-279.
-
- Brencic, A., Augert, E. R. & Winans, S. C. (2005) Mol. Microbiol. 57, 1522-1531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

