Species-specific differences in mouse and human airway epithelial biology of recombinant adeno-associated virus transduction
- PMID: 16195538
- PMCID: PMC1752084
- DOI: 10.1165/rcmb.2005-0189OC
Species-specific differences in mouse and human airway epithelial biology of recombinant adeno-associated virus transduction
Abstract
Differences in airway epithelial biology between mice and humans have presented challenges to evaluating gene therapies for cystic fibrosis (CF) using murine models. In this context, recombinant adeno-associated virus (rAAV) type 2 and rAAV5 vectors have very different transduction efficiencies in human air-liquid interface (ALI) airway epithelia (rAAV2 approximately = rAAV5) as compared with mouse lung (rAAV5 >> rAAV2). It is unclear if these differences are due to species-specific airway biology or limitations of ALI cultures to reproduce in vivo airway biology. To this end, we compared rAAV2 and rAAV5 transduction biology in mouse and human ALI cultures, and investigated the utility of murine deltaF508 cystic fibrosis transmembrane conductance regulator (CFTR) ALI epithelia to study CFTR complementation. Our results demonstrate that mouse ALI epithelia retain in vivo preferences for rAAV serotype transduction from the apical membrane (rAAV5 >> rAAV2) not seen in human epithelia (rAAV2 approximately = rAAV5). Viral binding of rAAV2 and rAAV5 to the apical surface of mouse ALI airway epithelia was not significantly different, and proteasome-modulating agents significantly enhanced rAAV2 transduction to a level equivalent to that of rAAV5 in the presence of these agents, suggesting that the ubiquitin/proteasome pathway represents a more significant intracellular block for rAAV2 transduction of mouse airway epithelia. Interestingly, cAMP-inducible chloride currents were enhanced in deltaF508CFTR mouse ALI cultures, making this model incompatible with CFTR complementation studies. These studies emphasize species-specific differences in airway biology between mice and humans that significantly influence the use of mice as surrogate models for rAAV transduction and gene therapy for CF.
Figures

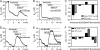




References
-
- Walters RW, Pilewski JM, Chiorini JA, Zabner J. Secreted and transmembrane mucins inhibit gene transfer with AAV4 more efficiently than AAV5. J Biol Chem 2002;277:23709–23713. - PubMed
-
- Sirninger J, Muller C, Braag S, Tang Q, Yue H, Detrisac C, Ferkol T, Guggino WB, Flotte TR. Functional characterization of a recombinant adeno-associated virus 5–pseudotyped cystic fibrosis transmembrane conductance regulator vector. Hum Gene Ther 2004;15:832–841. - PubMed
-
- Sarkar R, Tetreault R, Gao G, Wang L, Bell P, Chandler R, Wilson JM, Kazazian HH Jr. Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood 2004;103:1253–1260. - PubMed
-
- Moss RB, Rodman D, Spencer LT, Aitken ML, Zeitlin PL, Waltz D, Milla C, Brody AS, Clancy JP, Ramsey B, et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest 2004;125:509–521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

