Transcriptional regulation of lysophosphatidic acid-induced interleukin-8 expression and secretion by p38 MAPK and JNK in human bronchial epithelial cells
- PMID: 16197369
- PMCID: PMC1360718
- DOI: 10.1042/BJ20050791
Transcriptional regulation of lysophosphatidic acid-induced interleukin-8 expression and secretion by p38 MAPK and JNK in human bronchial epithelial cells
Abstract
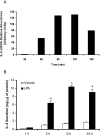
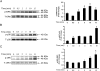
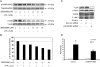
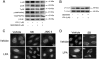
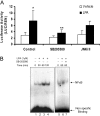
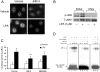
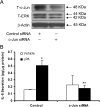
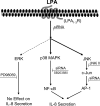
HBEpCs (human bronchial epithelial cells) contribute to airway inflammation by secreting a variety of cytokines and chemokines in response to allergens, pathogens, viruses and environmental toxins and pollutants. The potent neutrophil chemoattractant, IL-8 (interleukin-8), is a major cytokine secreted by HBEpCs. We have recently demonstrated that LPA (lysophosphatidic acid) stimulated IL-8 production in HBEpCs via protein kinase C delta dependent signal transduction. However, mechanisms of IL-8 expression and secretion are complex and involve multiple protein kinases and transcriptional factors. The present study was undertaken to investigate MAPK (mitogen-activated protein kinase) signalling in the transcriptional regulation of IL-8 expression and secretion in HBEpCs. Exposure of HBEpCs to LPA (1 microM) enhanced expression and secretion of IL-8 by 5-8-fold and stimulated threonine/tyrosine phosphorylation of ERK (extracellular-signal-regulated kinase), p38 MAPK and JNK (c-Jun N-terminal kinase). The LPA-induced secretion of IL-8 was blocked by the p38 MAPK inhibitor SB203580, by p38 MAPK siRNA (small interfering RNA), and by the JNK inhibitor JNK(i) II, but not by the MEK (MAPK/ERK kinase) inhibitor, PD98059. LPA enhanced the transcriptional activity of the IL-8 gene; that effect relied on activation of the transcriptional factors NF-kappaB (nuclear factor kappaB) and AP-1 (activator protein-1). Furthermore, SB203580 attenuated LPA-dependent phosphorylation of IkappaB (inhibitory kappaB), NF-kappaB and phospho-p38 translocation to the nucleus, NF-kappaB transcription and IL-8 promoter-mediated luciferase reporter activity, without affecting the JNK pathway and AP-1 transcription. Similarly, JNK(i) II only blocked LPA-mediated phosphorylation of JNK and c-Jun, AP-1 transcription and IL-8 promoter-mediated luciferase reporter activity, without blocking p38 MAPK-dependent NF-kappaB transcription. Additionally, siRNA for LPA(1-3) receptors partially blocked LPA-induced IL-8 production and activation of MAPKs. The LPA1 and LPA3 receptors, as compared with LPA2, were most efficient in transducing LPA-mediated IL-8 production. These results show an independent role for p38 MAPK and JNK in LPA-induced IL-8 expression and secretion via NF-kappaB and AP-1 transcription respectively in HBEpCs.
Figures
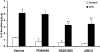
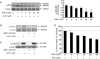

Similar articles
-
Lipid phosphate phosphatase-1 regulates lysophosphatidic acid-induced calcium release, NF-kappaB activation and interleukin-8 secretion in human bronchial epithelial cells.Biochem J. 2005 Jan 15;385(Pt 2):493-502. doi: 10.1042/BJ20041160. Biochem J. 2005. PMID: 15461590 Free PMC article.
-
Mitogen-activated protein kinases and nuclear factor-kappaB regulate Helicobacter pylori-mediated interleukin-8 release from macrophages.Biochem J. 2002 Nov 15;368(Pt 1):121-9. doi: 10.1042/BJ20020555. Biochem J. 2002. PMID: 12150710 Free PMC article.
-
Protein kinase Cdelta mediates lysophosphatidic acid-induced NF-kappaB activation and interleukin-8 secretion in human bronchial epithelial cells.J Biol Chem. 2004 Sep 24;279(39):41085-94. doi: 10.1074/jbc.M404045200. Epub 2004 Jul 26. J Biol Chem. 2004. PMID: 15280372
-
TNF-alpha/IL-1/NF-kappaB transduction pathway in human cancer prostate.Histol Histopathol. 2008 Oct;23(10):1279-90. doi: 10.14670/HH-23.1279. Histol Histopathol. 2008. PMID: 18712680 Review.
-
Human Protein Kinases and Obesity.Adv Exp Med Biol. 2017;960:111-134. doi: 10.1007/978-3-319-48382-5_5. Adv Exp Med Biol. 2017. PMID: 28585197 Review.
Cited by
-
Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation.Am J Respir Crit Care Med. 2013 Oct 15;188(8):928-40. doi: 10.1164/rccm.201306-1014OC. Am J Respir Crit Care Med. 2013. PMID: 24050723 Free PMC article.
-
Differential Effects of Inhibitor Combinations on Lysophosphatidic Acid-Mediated Chemokine Secretion in Unprimed and Tumor Necrosis Factor-α-Primed Synovial Fibroblasts.Front Pharmacol. 2017 Nov 21;8:848. doi: 10.3389/fphar.2017.00848. eCollection 2017. Front Pharmacol. 2017. PMID: 29209219 Free PMC article.
-
Lysophosphatidic acid signaling in airway epithelium: role in airway inflammation and remodeling.Cell Signal. 2009 Mar;21(3):367-77. doi: 10.1016/j.cellsig.2008.10.010. Epub 2008 Oct 26. Cell Signal. 2009. PMID: 18996473 Free PMC article. Review.
-
Autotaxin in Pathophysiology and Pulmonary Fibrosis.Front Med (Lausanne). 2018 Jun 13;5:180. doi: 10.3389/fmed.2018.00180. eCollection 2018. Front Med (Lausanne). 2018. PMID: 29951481 Free PMC article. Review.
-
Mice with targeted inactivation of ppap2b in endothelial and hematopoietic cells display enhanced vascular inflammation and permeability.Arterioscler Thromb Vasc Biol. 2014 Apr;34(4):837-45. doi: 10.1161/ATVBAHA.113.302335. Epub 2014 Feb 6. Arterioscler Thromb Vasc Biol. 2014. PMID: 24504738 Free PMC article.
References
-
- Matsukura S., Kokubu F., Noda H., Tokunaga H., Adach I. M. Expression of IL-6, IL-8, and RANTES on human bronchial epithelial cells, NCI-H292, induced by influenza virus A. J. Allergy Clin. Immunol. 1996;98:1080–1087. - PubMed
-
- Mio T., Romberger D. J., Thompson A. B., Robbins R. A., Heires A., Rennard S. I. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am. J. Respir. Crit. Care Med. 1997;155:1770–1776. - PubMed
-
- Johnston S. I., Papi A., Bates P. J., Mastronarde J. G., Monick M. M., Hunninghake G. W. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J. Immunol. 1998;160:6172–6181. - PubMed
-
- Hashimoto S., Gon Y., Takeshita I., Matsumoto K., Jibiki I., Takizawa H., Kudoh S., Horie T. Diesel exhaust particles activates p38 MAP kinase to produce interleukin 8 and RANTES by human bronchial epithelial cells and N-acetylcysteine attenuates p38 MAP kinase activation. Am. J. Respir. Crit. Care Med. 2000;161:280–285. - PubMed
-
- Aggarwal A., Baker C. S., Evans T. W., Haslam P. L. G-CSF and IL-8 but not GM-CSF correlates with severity of pulmonary neutrophilia in acute respiratory distress syndrome. Eur. Respir. J. 2000;15:895–901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

