Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury
- PMID: 16198706
- PMCID: PMC3529572
- DOI: 10.1016/S0079-6123(05)52017-X
Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury
Abstract
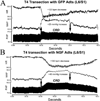
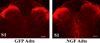
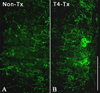
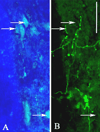
Spinal cord injuries above mid-thoracic levels can lead to a potentially life-threatening hypertensive condition termed autonomic dysreflexia that is often triggered by distension of pelvic viscera (bladder or bowel). This syndrome is characterized by episodic hypertension due to sudden, massive discharge of sympathetic preganglionic neurons in the thoracolumbar spinal cord. This hypertension is usually accompanied by bradycardia, particularly if the injury is caudal to the 2nd to 4th thoracic spinal segments. The development of autonomic dysreflexia is correlated with aberrant sprouting of peptidergic afferent fibers into the spinal cord below the injury. In particular, sprouting of nerve growth factor-responsive afferent fibers has been shown to have a major influence on dysreflexia, perhaps by amplifying the activation of disinhibited sympathetic neurons. Using a model of noxious bowel distension after complete thoracic spinal transection at the 4th thoracic segment in rats, we selectively altered C-fiber sprouting, at specified spinal levels caudal to the injury, with microinjections of adenovirus encoding the growth-promoting nerve growth factor or the growth-inhibitory semaphorin 3A. This was followed by assessment of physiological responses to colorectal distension and subsequent histology. Additionally, anterograde tract tracers were injected into the lumbosacral region to compare the extent of labeled propriospinal rostral projections in uninjured cords to those in cords after complete 4th thoracic transection. In summary, overexpression of chemorepulsive semaphorin 3A impeded C-fiber sprouting in lumbosacral segments and mitigated hypertensive autonomic dysreflexia, whereas the opposite results were obtained with nerve growth factor overexpression. Furthermore, compared to naïve rats, there were significantly more labeled lumbosacral propriospinal projections rostrally after thoracic injury. Collectively, our findings suggest that distension of pelvic viscera increases the excitation of expanded afferent terminals in the disinhibited lumbosacral spinal cord. This, in turn, triggers excitation and sprouting of local propriospinal neurons to relay visceral sensory stimuli and amplify the activation of sympathetic preganglionic neurons in the thoracolumbar cord, to enhance transmission in the spinal viscero-sympathetic reflex pathway. These responses are manifested as autonomic dysreflexia.
Figures
Similar articles
-
Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia.J Neurosci. 2006 Mar 15;26(11):2923-32. doi: 10.1523/JNEUROSCI.4390-05.2006. J Neurosci. 2006. PMID: 16540569 Free PMC article.
-
Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord.J Neurotrauma. 2001 Oct;18(10):1107-19. doi: 10.1089/08977150152693782. J Neurotrauma. 2001. PMID: 11686496
-
Plasticity of lumbosacral propriospinal neurons is associated with the development of autonomic dysreflexia after thoracic spinal cord transection.J Comp Neurol. 2008 Aug 1;509(4):382-99. doi: 10.1002/cne.21771. J Comp Neurol. 2008. PMID: 18512692 Free PMC article.
-
Autonomic dysreflexia after spinal cord injury: central mechanisms and strategies for prevention.Prog Brain Res. 2006;152:245-63. doi: 10.1016/S0079-6123(05)52016-8. Prog Brain Res. 2006. PMID: 16198705 Review.
-
Autonomic dysreflexia after spinal cord injury: Systemic pathophysiology and methods of management.Auton Neurosci. 2018 Jan;209:59-70. doi: 10.1016/j.autneu.2017.05.002. Epub 2017 May 8. Auton Neurosci. 2018. PMID: 28506502 Free PMC article. Review.
Cited by
-
Intraspinal Plasticity Associated With the Development of Autonomic Dysreflexia After Complete Spinal Cord Injury.Front Cell Neurosci. 2019 Nov 8;13:505. doi: 10.3389/fncel.2019.00505. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31780900 Free PMC article.
-
Brain-Dependent Processes Fuel Pain-Induced Hemorrhage After Spinal Cord Injury.Front Syst Neurosci. 2019 Sep 10;13:44. doi: 10.3389/fnsys.2019.00044. eCollection 2019. Front Syst Neurosci. 2019. PMID: 31551720 Free PMC article.
-
Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia.J Neurosci. 2006 Mar 15;26(11):2923-32. doi: 10.1523/JNEUROSCI.4390-05.2006. J Neurosci. 2006. PMID: 16540569 Free PMC article.
-
Dual-directional regulation of spinal cord injury and the gut microbiota.Neural Regen Res. 2024 Mar;19(3):548-556. doi: 10.4103/1673-5374.380881. Neural Regen Res. 2024. PMID: 37721283 Free PMC article. Review.
-
Gastric dysreflexia after acute experimental spinal cord injury in rats.Neurogastroenterol Motil. 2009 Feb;21(2):197-206. doi: 10.1111/j.1365-2982.2008.01215.x. Epub 2008 Dec 19. Neurogastroenterol Motil. 2009. PMID: 19126185 Free PMC article.
References
-
- Al-Chaer ED, Westlund KN, Willis WD. Sensitization of postsynaptic dorsal column neuronal responses by colon inflammation. Neuroreport. 1997;8:3267–3273. - PubMed
-
- Al-Chaer ED, Feng Y, Willis WD. Comparative study of viscerosomatic input onto postsynaptic dorsal column and spinothalamic tract neurons in the primate. J. Neurophysiol. 1999;82:1876–1882. - PubMed
-
- Al-Chaer ED, Lawand NB, Westlund KN, Willis WD. Pelvic visceral input into the nucleus gracilis is largely mediated by the postsynaptic dorsal column pathway. J. Neurophysiol. 1996;76:2675–2690. - PubMed
-
- Anderson CR, Edwards SL. Intraperitoneal injections of Fluorogold reliably labels all sympathetic preganglionic neurons in the rat. J. Neurosci. Methods. 1994;53:137–141. - PubMed
-
- Bahr R, Bartel B, Blumberg H, Janig W. Functional characterization of preganglionic neurons projecting in the lumbar splanchnic nerves: vasoconstrictor neurons. J. Auton. Nerv. Syst. 1986;15:131–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

