5-Hydroxytryptamine-receptor antagonists versus prochlorperazine for control of delayed nausea caused by doxorubicin: a URCC CCOP randomised controlled trial
- PMID: 16198982
- PMCID: PMC1646426
- DOI: 10.1016/S1470-2045(05)70325-9
5-Hydroxytryptamine-receptor antagonists versus prochlorperazine for control of delayed nausea caused by doxorubicin: a URCC CCOP randomised controlled trial
Abstract
Background: Despite widespread use of short-acting antagonists for the 5-hydroxytryptamine (5-HT) receptor, about 50% of patients given moderately emetogenic chemotherapy have delayed nausea. We aimed to assess whether a 5-HT-receptor antagonist was more effective than was prochlorperazine for control of delayed nausea and delayed vomiting caused by doxorubicin.
Methods: 691 patients who previously had not had chemotherapy and who were scheduled to receive doxorubicin were given a short-acting 5-HT-receptor antagonist and dexamethasone before doxorubicin (day 1), and were randomly assigned to one of three regimens for days 2 and 3: 10 mg prochlorperazine taken orally every 8 h; any first-generation 5-HT-receptor antagonist (except palonosetron) taken as standard dose intravenously or orally; or 10 mg prochlorperazine taken as needed. Nausea and vomiting were assessed by use of a home record. The primary endpoint was mean severity of delayed nausea. The secondary endpoint was quality of life. Analyses were done by intention to treat.
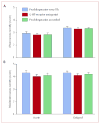
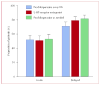
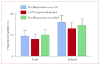
Findings: 519 (77%) of the 671 evaluable patients had delayed nausea, with a mean severity of 3.33 (95% CI 3.22-3.44). 161 (71%) of 226 patients assigned prochlorperazine every 8 h reported delayed nausea (mean severity 3.37 [3.16-3.58]), as did 179 (79%) of 226 patients assigned 5-HT-receptor antagonists (3.29 [3.09-3.48]) and 179 (82%) of 219 patients assigned prochlorperazine as needed (3.33 [3.15-3.50]); groups did not differ in mean severity (p=0.853, one-way ANOVA). Patients allocated prochlorperazine every 8 h had less delayed nausea than did those allocated 5-HT-receptor antagonists (p=0.05, t test) and those allocated prochlorperazine as needed (p=0.009, t test).
Interpretation: Short-acting 5-HT-receptor antagonists are no better than is prochlorperazine in control of delayed nausea caused by doxorubicin. Although fewer patients taking prochlorperazine report delayed nausea, the proportion was unacceptably high.
Figures


Similar articles
-
Prevention of delayed chemotherapy-induced nausea and vomiting after moderately high to highly emetogenic chemotherapy: comparison of ondansetron, prochlorperazine, and dexamethasone.Am J Clin Oncol. 2005 Jun;28(3):270-6. doi: 10.1097/01.coc.0000145983.35929.2a. Am J Clin Oncol. 2005. PMID: 15923800 Clinical Trial.
-
Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial.Lancet Oncol. 2009 Feb;10(2):115-24. doi: 10.1016/S1470-2045(08)70313-9. Epub 2009 Jan 8. Lancet Oncol. 2009. PMID: 19135415 Clinical Trial.
-
Effectiveness of a single-day three-drug regimen of dexamethasone, palonosetron, and aprepitant for the prevention of acute and delayed nausea and vomiting caused by moderately emetogenic chemotherapy.Support Care Cancer. 2009 May;17(5):589-94. doi: 10.1007/s00520-008-0535-9. Epub 2008 Nov 27. Support Care Cancer. 2009. PMID: 19037667 Clinical Trial.
-
Palonosetron: an evidence-based choice in prevention of nausea and vomiting induced by moderately emetogenic chemotherapy.Tumori. 2012 May-Jun;98(3):279-86. doi: 10.1177/030089161209800301. Tumori. 2012. PMID: 22825501 Review.
-
Palonosetron: a unique 5-HT3 receptor antagonist indicated for the prevention of acute and delayed chemotherapy-induced nausea and vomiting.Clin Adv Hematol Oncol. 2004 May;2(5):284-9. Clin Adv Hematol Oncol. 2004. PMID: 16163194 Review.
Cited by
-
Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systematic review and meta-analysis.Support Care Cancer. 2011 Jun;19(6):823-32. doi: 10.1007/s00520-010-0908-8. Epub 2010 May 22. Support Care Cancer. 2011. PMID: 20495832
-
ASCO, NCCN, MASCC/ESMO: a comparison of antiemetic guidelines for the treatment of chemotherapy-induced nausea and vomiting in adult patients.Support Care Cancer. 2019 Jan;27(1):87-95. doi: 10.1007/s00520-018-4464-y. Epub 2018 Oct 3. Support Care Cancer. 2019. PMID: 30284039 Review.
-
Pooled analysis of phase III clinical studies of palonosetron versus ondansetron, dolasetron, and granisetron in the prevention of chemotherapy-induced nausea and vomiting (CINV).Support Care Cancer. 2014 Feb;22(2):469-77. doi: 10.1007/s00520-013-1999-9. Epub 2013 Oct 19. Support Care Cancer. 2014. PMID: 24141698 Free PMC article. Clinical Trial.
-
Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: results of a randomized, double-blind phase III trial.Ann Oncol. 2016 Jan;27(1):172-8. doi: 10.1093/annonc/mdv482. Epub 2015 Oct 8. Ann Oncol. 2016. PMID: 26449391 Free PMC article. Clinical Trial.
-
Combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy in the SENRI trial: analysis of risk factors for vomiting and nausea.Int J Clin Oncol. 2017 Feb;22(1):88-95. doi: 10.1007/s10147-016-1022-9. Epub 2016 Jul 27. Int J Clin Oncol. 2017. PMID: 27465476 Clinical Trial.
References
-
- Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261–68. - PubMed
-
- Hickok JT, Roscoe JA, Morrow GR, et al. Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics: A University of Rochester James P Wilmot Cancer Center Community Clinical Oncology Program Study of 360 cancer patients treated in the community. Cancer. 2003;97:2880–86. - PubMed
-
- Gralla RJ, Osoba D, Kris MG, et al. Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society of Clinical Oncology. J Clin Oncol. 1999;17:2971–94. - PubMed
-
- Kris MG, Hesketh PJ, Herrstedt J, et al. Consensus proposals for the prevention of acute and delayed vomiting and nausea following high-emetic-risk chemotherapy. Support Care Cancer. 2005;13:85–96. - PubMed
-
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology—antiemesis, v.1.2005. http://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf (accessed Aug 24, 2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

