5-Hydroxytryptamine-receptor antagonists versus prochlorperazine for control of delayed nausea caused by doxorubicin: a URCC CCOP randomised controlled trial
- PMID: 16198982
- PMCID: PMC1646426
- DOI: 10.1016/S1470-2045(05)70325-9
5-Hydroxytryptamine-receptor antagonists versus prochlorperazine for control of delayed nausea caused by doxorubicin: a URCC CCOP randomised controlled trial
Abstract
Background: Despite widespread use of short-acting antagonists for the 5-hydroxytryptamine (5-HT) receptor, about 50% of patients given moderately emetogenic chemotherapy have delayed nausea. We aimed to assess whether a 5-HT-receptor antagonist was more effective than was prochlorperazine for control of delayed nausea and delayed vomiting caused by doxorubicin.
Methods: 691 patients who previously had not had chemotherapy and who were scheduled to receive doxorubicin were given a short-acting 5-HT-receptor antagonist and dexamethasone before doxorubicin (day 1), and were randomly assigned to one of three regimens for days 2 and 3: 10 mg prochlorperazine taken orally every 8 h; any first-generation 5-HT-receptor antagonist (except palonosetron) taken as standard dose intravenously or orally; or 10 mg prochlorperazine taken as needed. Nausea and vomiting were assessed by use of a home record. The primary endpoint was mean severity of delayed nausea. The secondary endpoint was quality of life. Analyses were done by intention to treat.
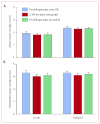
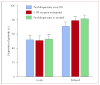
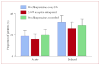
Findings: 519 (77%) of the 671 evaluable patients had delayed nausea, with a mean severity of 3.33 (95% CI 3.22-3.44). 161 (71%) of 226 patients assigned prochlorperazine every 8 h reported delayed nausea (mean severity 3.37 [3.16-3.58]), as did 179 (79%) of 226 patients assigned 5-HT-receptor antagonists (3.29 [3.09-3.48]) and 179 (82%) of 219 patients assigned prochlorperazine as needed (3.33 [3.15-3.50]); groups did not differ in mean severity (p=0.853, one-way ANOVA). Patients allocated prochlorperazine every 8 h had less delayed nausea than did those allocated 5-HT-receptor antagonists (p=0.05, t test) and those allocated prochlorperazine as needed (p=0.009, t test).
Interpretation: Short-acting 5-HT-receptor antagonists are no better than is prochlorperazine in control of delayed nausea caused by doxorubicin. Although fewer patients taking prochlorperazine report delayed nausea, the proportion was unacceptably high.
Figures


References
-
- Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261–68. - PubMed
-
- Hickok JT, Roscoe JA, Morrow GR, et al. Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics: A University of Rochester James P Wilmot Cancer Center Community Clinical Oncology Program Study of 360 cancer patients treated in the community. Cancer. 2003;97:2880–86. - PubMed
-
- Gralla RJ, Osoba D, Kris MG, et al. Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society of Clinical Oncology. J Clin Oncol. 1999;17:2971–94. - PubMed
-
- Kris MG, Hesketh PJ, Herrstedt J, et al. Consensus proposals for the prevention of acute and delayed vomiting and nausea following high-emetic-risk chemotherapy. Support Care Cancer. 2005;13:85–96. - PubMed
-
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology—antiemesis, v.1.2005. http://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf (accessed Aug 24, 2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

